
Concept explainers
(a)
Interpretation:
Whether the following Neman Projection is a representation of the molecule in the previous question should be determined.
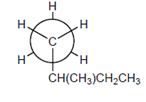
Concept Introduction:
A diagram which is used to denote the specific conformation of a molecule is known as Newman projection. It is classified as: Staggered conformation and Eclipsed conformation.
(b)
Interpretation:
The carbons 1-5 should be labeled on the following Newman projection.
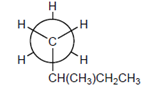
Concept Introduction:
A diagram which is used to denote the specific conformation of a molecule is known as Newman projection. It is classified as: Staggered conformation and Eclipsed conformation.
(c)
Interpretation:
The Lewis structure of the given Newman projection should be drawn.
Concept Introduction:
A diagram which is used to denote the specific conformation of a molecule is known as Newman projection. It is classified as: Staggered conformation and Eclipsed conformation.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Organic Chemistry: A Guided Inquiry
- a model of each molecule shown above: Is the molecule in the left box the same moleculeas the molecule in the right box? Use your models to answer the question, and recall that...arrow_forward8. If a resonance structure must have a negative formal charge, that charge is most stable on an electronegative atom. Draw all relevant resonance structures of the molecule below with curved arrows only using pattern 1. Circle the most stable structure(s). Explain the choice of most stable structure with 1-3 complete sentences.arrow_forwardCould we cut just one bond in the "starting" molecule shown in the drawing area below to create this "target" molecule? The target molecule. If so, highlight the bond to be cut. If not, check the box under the drawing area that says Not possible. Note: it's OK if cutting the bond creates more than one molecule, as long as one of them is the target molecule. Not possible. X H H-C-H H-O-H H 3 Note for advanced students: what we mean by "cutting" the bond here is breaking the bond and attaching H atoms to each dangling end, like this: H H-C-0-H Harrow_forward
- e. Draw the cis and trans isomer of the molecule above. f. Draw the chair form of the cis isomer of the molecule. g. Draw the flipped chair form of the cis isomer of the molecule. h. What is the more stable chair form?arrow_forwardWithout rotating the bond, draw a proper Newman structure of the following molecule, looking down the bond in the direction of the arrow. [Select] [Select] OH Br [Select] [ Select ]arrow_forward3. Ror S determination. For each molecule below, determine if it is in the R or S configuration for the carbon atoms with the * using the rules discussed in class (and in the book), Write the configuration on the line BELOW the compound. HO H H. Br HN- CEN CF3 SHarrow_forward
- b. On the same figure, label all positions that are gauche to the Y group on carbon 1. A bin may hold more than one label. # 4arrow_forward#11 - I need help with only the second row. Row 1 and 3 are correect. The answer is not -11 for row 2. Please help.arrow_forward2. Each resonance structure of a given molecule contributes to the resonance hybrid. The more stable the resonance structure, the more that resonance structure contributes to the resonance hybrid. For the following resonance structures, a. Circle the resonance structure that contributes most to the resonance hybrid. b. Explain your choice for a in complete sentences. c. Draw the resonance hybrid. d. Add curved arrows to indicate the flow of electrons in each structure except the D. H A Q Search B hyji C Darrow_forward
- CH3 OH a. Draw the cis and trans isomer of the molecule above. b. Draw the chair form of the trans isomer of the molecule. c. Challenge: Draw the flipped chair form of the trans isomer of the molecule.arrow_forwardHi, can you please add or indicate the lone pairs just like the second drawing (digital)? I also need a short explanation for each number.arrow_forward4. Circle the following pairs of structures that do not constitute resonance structures. For the proper resonance pairs, draw curved arrows to convert the first structure to the second. Draw in all lone pairs of electrons. H3C-CH3 „CH3 HgC-N' a) b) H2C=C=CH2 H3C-CECH FCH2 d) H3C' CH2 H H3Carrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
