
Concept explainers
Using your model of butane
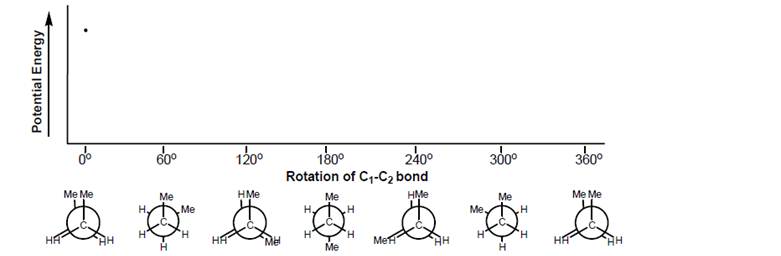 a. Label each Newman projection of butane on the graph with the words staggered, eclipsed, gauche, and anti, as appropriate. (Note that some structures will have more than one label.)
a. Label each Newman projection of butane on the graph with the words staggered, eclipsed, gauche, and anti, as appropriate. (Note that some structures will have more than one label.)
b. Draw a wedge and dash bond representation of butane in its lowest P.E. conformation.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Organic Chemistry: A Guided Inquiry
- Q2. a) Draw the Newman projection of the most stable staggered conformation of 2-methylpentane, looking down the 3,4 bond. H3C c-CH2-CH2CH3 H3C´H b) The structure of cis-1-tert-butyl-3-methylcyclohexane is shown below. Draw both chair forms of this molecule, and circle the one you think is less stable. Me 't-Buarrow_forwardH3C H. NHCH3 NHCH3 H Harrow_forward1. Draw the most and least stable Newman projections for the following molecules (focus on C2 and C3). a. 2-chloro-2-fluoropentane b. 2,2-dimethylbutane c. 2-chloro-2-methylpentane d. 1,2-dibromoethanearrow_forward
- Draw the most and least stable Newman projections for the following molecules ( focus on C2 and C3). a. 2-chloro-2- fluoropentane b. 2,2-dimethylbutane C. 2-chloro-2-methylpentane d. 1,2-dibromoethanearrow_forwardC. Using your molecular modeling kit, construct cis-1,4-dimethylcyclohexane. In the space below, draw one of the chair conformations. Label the methyl groups as either axial (a) or equatorial (e). Number the carbons of the cyclohexane ring, perform a chair flip and draw the new chair conformation.arrow_forward3. For pentane draw Newman projections for the Syn-periplanar, conformation. the Anti- periplanar conformation and a Gauche conformation. Use C2 as the front carbon and C3 as the back carbon. Label each conformation, circle the highest energy conformation and underline the lowest energy conformation.arrow_forward
- DIRECTIONS:Provide the required number of isomers ner molecular formula by illustrating them in skeletal structure. Afterwards, identify the IUPAC name of each isomer. 1. C,H,0 (4 isomers) 2. C,H,0, (4 isomers) 3. C,H,O (3 isomers) DIRECTIONS. Answer the following questions in complete sentences. 1. What specific type of isomer/s are in Part 1: item 3? 2. What type of organic compound/s have you drawn in Part 2: Item 2? 3. Draw a pair of enantiomers and encircle its chiral carbon.arrow_forwardSight along the C2-Cl bond of 2-methylpropane (isobutane). a. Draw a Newman projection of the most stable conformation. b. Draw a Newman projection of the least stable conformation. c. Make a graph of energy versus angle of rotation around the C2-Cl bond. d. Assign relative values to the maxima and minima in your graph, given that an H↔H eclipsing interaction costs 0 kJ/mol and an H↔CH3 eclipsing interaction costs 6.0 kJ/mol.arrow_forward1: Draw the chain conformer of... 1a. Cyclohexane, label all the axial and equatorial hydrogens 1b. The most stable conformer of ethylcyclohexane 1c. The most sable conformer of trans-1-tert-butyl-3-methylcyclohexanearrow_forward
- 5. Chairs and E2 b. a. Draw the line-bond structure of (1R, 2S,3S)-1-ethyl-2-iodo-3-isopropylcyclohexane. Draw both chair flips of (1R, 2S,3S)-1-ethyl-2-iodo-3-isopropylcyclohexane. Showing all calculations, determine which is the more stable conformation. C. Which chair flip is able to undergo E2 elimination? Justify your answer. d. Show the product of such an E2 elimination using NaOEt as base.arrow_forwardConsider the cyclohexane framework in a chair conformation, where carbon 1 has two substituents, X and Y. a. Label each position as axial or equatorial. b. On the same figure, label all positions that are gauche to the Y group on carbon 1. A bin may hold more than one label. Answer Bank equatorial axial guachearrow_forwardConsider the cyclohexane framework in a chair conformation, where carbon 1 has two substituents, X and Y. a. Label each position as axial or equatorial. b. On the same figure, label all positions that are gauche to the Y group on carbon 1. A bin may hold more than one label. axial equatorial X 1 equatorial equatorial 2 axial axial axial gauche 3 axial gauche 5 equatorial equatorial 4 axial equatorial Answer Bank equatorial gauche axialarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
