
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
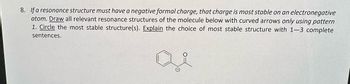
Transcribed Image Text:**Question 8:**
If a resonance structure must have a negative formal charge, that charge is most stable on an electronegative atom.
**Instructions:**
1. Draw all relevant resonance structures of the molecule below with curved arrows only using pattern.
2. Circle the most stable structure(s).
3. Explain the choice of most stable structure with 1–3 complete sentences.
**Diagram:**
The diagram displays a benzene ring attached to an acetyl group, which is a carbon double-bonded to oxygen and single-bonded to a methyl group. The oxygen can carry a negative charge when depicting resonance structures.
---
This instructional material helps you understand how to identify and illustrate resonance structures while considering the stability of negative charges on electronegative atoms, such as oxygen.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Let's Try (Evaluation) Match each compound whose Lewis structural formula is given below as one of the following functional groups (fil in the number) 1. Alcohol Ether 2. Aldehyde 3. 4. Ketone 5 Amine 6. Carboxylic Acid 7. Ester 8. Amidearrow_forwardXeF4arrow_forwardThere are three different possible structures (known as isomers) of a dibromoethene molecule, C, H,Br,. One of them has no net dipole moment, but the other two do. Draw Lewis structures for each of these structures. Include H atoms. nonpolar C, H,Br, structure (one structure) Select Draw Rings More Erase C H Br Qarrow_forward
- 2. Each resonance structure of a given molecule contributes to the resonance hybrid. The more stable the resonance structure, the more that resonance structure contributes to the resonance hybrid. For the following resonance structures, a. Circle the resonance structure that contributes most to the resonance hybrid. b. Explain your choice for a in complete sentences. c. Draw the resonance hybrid. d. Add curved arrows to indicate the flow of electrons in each structure except the D. H A Q Search B hyji C Darrow_forward! ( plz answer with explanation)arrow_forwardTwo resonance structures are possible for the anion shown. One resonance form is given, but it is incomplete. Complete the given structure by adding nonbonding electrons and formal charges. Draw the remaining resonance structure, including nonbonding electrons and formal charges. Omit curved arrows. Structure A: complete the structure by adding Structure B: draw the remaining resonance structure, nonbonding electrons and formal charges. including nonbonding electrons and formal charges. Rings Erase Select Draw Rings More Erase Select Draw More H // C H. Harrow_forward
- 2. Tetrahydrocannabinolic acid (THCA), the precursor to THC, a psvchoactive: H.. • This molecule is missing eight lone pairs. Add them to the structure above • Check the formal charges of the oxygen atoms above. Do any of them have a non-zero formal charge? If so, add the charges to the appropriate oxygen atoms • THCA is a weak acid. Identify and circle the acidic hydrogen on the above structure • Draw a resonance structure of this molecule belowarrow_forwardOne, or both, of the following structural formulas may be incorrect (i. e., they do not represent a real compound) because they have atoms with an incorrect number of bonds. Which of the labeled atoms have the incorrect number of bonds? Specify the incorrect atoms by letter in alphabetical order without spaces or commas., i.e. bd. If there are no incorrect atoms, write none. a) b) H H lb lc d -C-0-H a H-N -C H bN H Harrow_forwarda) Draw the Lewis structure for the molecule on the left (labeled as Molecule A above). Draw the Lewis structure which has minimum formal charges. b) Draw the correct Lewis structure for the molecule on the right (labeled as Molecule B above). Draw the Lewis structure which has minimum formal charges. c) Select the three TRUE statements from those provided below. The molecule on the right (Molecule B) is planar (all atoms lie within the same plane). The molecule on the left (Molecule A) is planar (all atoms lie within the same plane). The molecule on the right (Molecule B) has polar bonds. The molecule on the left (Molecule A) has polar bonds. We can distinguish between the two molecules (Molecule A and Molecule B) based upon their dipole moment.arrow_forward
- Tag all the carbon atoms with pi bonds in this molecule. If there are none, please check the box below. H | || :0: H-C- I H .. C-O-H There are none. Xarrow_forwardWhich of the following species is a valid resonance structure of A? Use curved arrows to show how A is converted to any valid resonance structure. When a compound is not a valid resonance structure of A, explain why not.arrow_forwardPlease send me answer of this question immediately i will give you like sure sirarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY