
a)
Interpretation : The frequencies of the given wavelengths are to be calculated.
Concept Introduction : The separation between the crests is known as the wavelength.
The frequency is how often waves cycle through a specific point in a unit of time.
The formula of frequency
Where
a)
Answer to Problem 81A
The frequency
The frequency
The frequency
The frequency
The frequency
The frequency
Explanation of Solution
Given information:
The wavelength
The wavelength
The wavelength
The wavelength
The wavelength
The wavelength
It is known that velocity of light is
The separation between the crests is known as the wavelength.
The frequency is how often waves cycle through a specific point in a unit of time.
The formula of frequency
is given as:
Where
To calculate the frequency
To calculate the frequency
To calculate the frequency
To calculate the frequency
To calculate the frequency
To calculate the frequency
b)
Interpretation : The energy of the photon and frequency are to be plotted.
Concept Introduction : Planck demonstrated mathematically that a body's ability to absorb or emit radiation is inversely related to the frequency of the radiation (v). The formula of energy is given as
Where
b)
Answer to Problem 81A
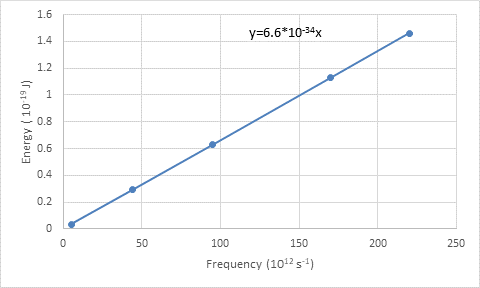
The energy of the photon is plotted on the y-axis while the frequency in the x-axis.
Explanation of Solution
The calculated frequencies and energy of the photon are tabulated.
| Frequency | Energy of photon |
| 5.20 | 345 |
| 44.0 | 29.2 |
| 94.90 | 62.9 |
| 170 | 1.13 |
| 220 | 1.46 |
| 470 | 3.11 |
The graph is plotted by frequency on the x-axis and energy of the photon on the y-axis. The plotting is done according to Planck’s energy formula.
c)
Interpretation : The energy of the photon and frequency is to be plotted.
Concept Introduction : Planck demonstrated mathematically that a body's ability to absorb or emit radiation is inversely related to the frequency of the radiation (v).
c)
Answer to Problem 81A
The slope of the line obtained is
Explanation of Solution
From the graph, the obtained equation is
According to the equation
d)
Interpretation : The energy of the photon and frequency is to be plotted.
Concept Introduction : The separation between the crests is known as the wavelength.
The frequency is how often waves cycle through a specific point in a unit of time.
d)
Answer to Problem 81A
The slope of the line obtained is Planck’s constant.
Explanation of Solution
The significance of the slope is Planck’s constant.
Planck demonstrated mathematically that a body's ability to absorb or emit radiation is inversely related to the frequency of the radiation (v). The formula of energy is given as
Where
Chapter 5 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





