
a.
Interpretation:The structural formula of 2,5-dimethyl-3-hexene is to be drawn.
Concept Introduction: The structural formula can be drawn from the name of a compound with the help of IUPAC principles.
a.
Answer to Problem 1RQ
The structural formula for 2,5-dimethyl-3-hexene is
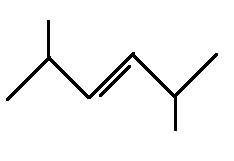
Explanation of Solution
The main chain is six carbons and the two branches represent methyl groups. The
b.
Interpretation:The structural formula of 4-methyl-2-pentene is to be drawn.
Concept Introduction: The structural formula can be drawn from the name of a compound with the help of IUPAC principles.
b.
Answer to Problem 1RQ
The structural formula for 4-methyl-2-pentene is
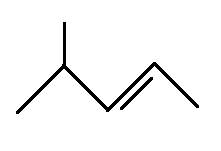
Explanation of Solution
The main chain is five carbons and the one branch represents methyl group. The alkene is on the second bond so the above structure is correct.
c.
Interpretation:The structural formula of iodoethyne is to be drawn.
Concept Introduction: The structural formula can be drawn from the name of a compound with the help of IUPAC principles.
c.
Answer to Problem 1RQ
The structural formula for iodoethyne is
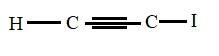
Explanation of Solution
The main chain is two carbons and the bond between them is triple which represents -yne. Also the I atom attached corresponds to iodo- prefix so the above structure is correct.
d.
Interpretation:The structural formula of 3-methylpentyne is to be drawn.
Concept Introduction: The structural formula can be drawn from the name of a compound with the help of IUPAC principles.
d.
Answer to Problem 1RQ
The structural formula for 3-methylpentyne is 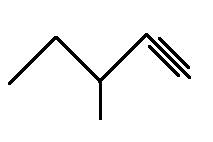
Explanation of Solution
The main chain is five carbons and the one branch on third carbon represents methyl group. The triple bond is on the first carbon representing -yne so the above structure is correct.
Chapter 20 Solutions
World of Chemistry, 3rd edition
- The Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forward
- Only 100% sure experts solve it correct complete solutions need to get full marks it's my quiz okkkk.take your time but solve full accurate okkk chemistry expert solve itarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardShow work. don't give Ai generated solutionarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





