
Concept explainers
(a)
Interpretation:
The structural formula of the compound is to be written.
Concept introduction:
To write the structural formula,
(a)
Answer to Problem 56A
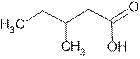
Explanation of Solution
The root name ‘pentan’ shows five carbon atoms in the main chain which are single bonded.
The name indicates one methyl group is attached at C-3 and C-1 is carboxylic group (
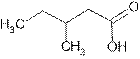
(b)
Interpretation:
The structural formula of the compound is to be written.
Concept introduction:
To write the structural formula, functional group, number of carbon atoms in the chain is to be identified.
(b)
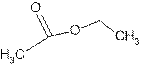
Answer to Problem 56A
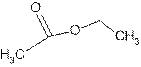
Explanation of Solution
The name ethyl methanoate shows compound contains ester functional group.
Its general formula is
The esters are name by taking alkyl chain from alcoholic part as substituent. Then name of parent chain of
(c)
Interpretation:
The structural formula of the compound is to be written.
Concept introduction:
To write the structural formula, functional group, number of carbon atoms in the chain is to be identified.
(c)
Answer to Problem 56A
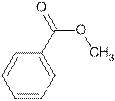
Explanation of Solution
The name Methyl benzoate shows compound contains ester functional group.
Its general formula is
The esters are name by taking alkyl chain from alcoholic part as substituent. Then name of parent chain of carboxylic acid is written by removing ‘e’ and adding ‘oate’. So the structure of Methyl benzoate is
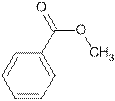
(d)
Interpretation:
The structural formula of the compound is to be written.
Concept introduction:
To write the structural formula, functional group, number of carbon atoms in the chain is to be identified.
(d)
Answer to Problem 56A
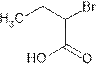
Explanation of Solution
The root name ‘butan’ shows four carbon atoms in the main chain which are single bonded.
The name indicates one bromo group is attached at C-2 and C-1 is carboxylic group (
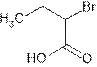
(e)
Interpretation:
The structural formula of the compound is to be written.
Concept introduction:
To write the structural formula, functional group, number of carbon atoms in the chain is to be identified.
(e)
Answer to Problem 56A
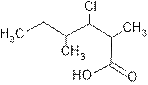
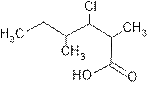
Explanation of Solution
The root name ‘hexan’ shows six carbon atoms in the main chain which are single bonded.
The name indicates two methyl groups are attached, one at C-2 and other at C-4 and one chloro is attached at C-3 and C-1 is carboxylic group (
Chapter 20 Solutions
World of Chemistry, 3rd edition
- Hi, I need help on my practice final, If you could offer strategies and dumb it down for me with an explanation on how to solve that would be amazing and beneficial.arrow_forwardHi I need help with my practice final, it would be really helpful to offer strategies on how to solve it, dumb it down, and a detailed explanation on how to approach future similar problems like this. The devil is in the details and this would be extremely helpfularrow_forwardIn alpha-NbI4, Nb4+ should have the d1 configuration (bond with paired electrons: paramagnetic). Please comment.arrow_forward
- Hi, I need help on my practice final, if you could explain how to solve it offer strategies and dumb it down that would be amazing. Detail helpsarrow_forwardBriefly explain the following paragraph: both the distortion of symmetry and the fact that the solid is diamagnetic indicate the existence of a Nb-Nb bond.arrow_forwardHi I need help on my practice final, If you could explain how to solve it, offer strategies, and dumb it down that would be amazing.arrow_forward
- -1 2 3 4 5 7 8 At a certain temperature this reaction follows first-order kinetics with a rate constant of 0.0635 s 2C1,0, (g) →2C1, (g)+50, (g) Suppose a vessel contains C1,0, at a concentration of 1.03 M. Calculate how long it takes for the concentration of C1,0, to decrease by 86.0%. You may assume no other reaction is important. Round your answer to 2 significant digits. e х th Earrow_forwardASAP....arrow_forwardNonearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





