
Concept explainers
Interpretation: The appropriate option that suits for the similarity of an ester to a
Concept introduction: Carboxylic acid consists of carboxyl group, which contains two
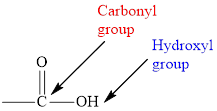
In carboxylic acid, the second oxygen atom is linked to a hydrogen atom.
The general formula for carboxylic acid is
Esters are carboxylic acid derivatives. The carbonyl group of the ester is polar. The general structure is,
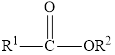
In an ester, the second oxygen atom is linked to another carbon atom.
The general formula for ester is
Answer to Problem 73A
An ester is similar to a carboxylic acid as a
Explanation of Solution
Carboxylic acid consists of carboxyl group, which contains two functional groups namely carbonyl and hydroxyl group.
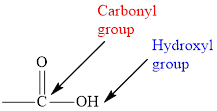
In carboxylic acid, the second oxygen atom is linked to a hydrogen atom.
The general formula for carboxylic acid is
Esters are carboxylic acid derivatives. The carbonyl group of the ester is polar and could take part in dipole-dipole attractions.
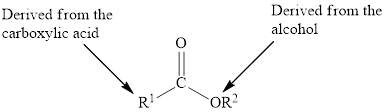
In an ester, the second oxygen atom is linked to another carbon atom.
The general formula for ester is
Ketones and aldehyde contain carbonyl group.
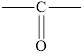
In ketones, the carbonyl group is bond to two carbon atoms. The general formula of a ketone is
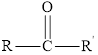
In a ketone, the carbonyl group is not present at the end of the hydrocarbon chain.
In aldehydes, the carbonyl group is attached at the end of the hydrocarbon chain. The carbonyl carbon is bonded to atleast one hydrogen.
Aldehydes have a general formula of
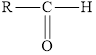
Here,
In an ester, an oxygen atom is attached to another carbon group bonded to the carbonyl carbon atom whereas carboxylic acid comprises of hydroxyl group attached to the carbonyl carbon atom.
In an aldehyde, one hydrogen atom is bonded to the carbonyl carbon atom whereas in ketone, two carbon groups are bonded to the carbonyl carbon atom.
An ester is similar to a carboxylic acid as a ketone is similar to an aldehyde. Option (e) is correct.
An ester is similar to a carboxylic acid is not like a primary alcohol similar to a secondary alcohol because in primary alcohol, the hydroxyl group is attached to single alkyl group whereas in secondary alcohol, the hydroxyl group is attached to two alkyl groups on either side.
The general formula of primary alcohol is
The general formula of secondary alcohol is
In an ester, an oxygen atom is attached to another carbon group bonded to the carbonyl carbon atom whereas carboxylic acid comprises of hydroxyl group attached to the carbonyl carbon atom.
The general formula for ester is
The general formula for carboxylic acid is
Here,
Option (a) is incorrect.
An ester is similar to a carboxylic acid is not like
The general formula of amine is
The general formula of ketone is
The general formula for ester is
The general formula for carboxylic acid is
Here,
Option (b) is incorrect.
An ester is similar to a carboxylic acid is not like ether similar to an aldehyde because ether (functional group containing oxygen atom bonded to two alkyl groups) does not a carbonyl carbon like aldehyde. Whereas an ester and carboxylic acid shows the presence of carbonyl carbon (in carboxylic acid, carbonyl carbon and hydroxyl group is collectively called as carboxyl group).
The general formula of ether is
The general formula of aldehyde is
The general formula for ester is
The general formula for carboxylic acid is
Here,
Option (c) is incorrect.
An ester is similar to a carboxylic acid is not like aromatic ring similar to a polymer because aromatic rings are hydrocarbons that contains benzene or some ring compounds. Polymers are repeated units of monomer, whereas an ester and carboxylic acid shows the presence of carbonyl carbon (in carboxylic acid, carbonyl carbon and hydroxyl group is collectively called as carboxyl group).
Option (d) is incorrect.
Chapter 20 Solutions
World of Chemistry, 3rd edition
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





