
Concept explainers
Interpretation:
The structural formula of
Concept Introduction:
The given compound is the branched chain
Answer to Problem 65A
The structural formula of
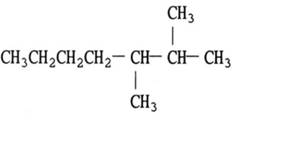
Explanation of Solution
- Select the longest continuous chain containing the carbon attached to the halogen group and this is the parent chain.
- The number of the carbon atoms of the parent chain, begging from the end nearer to the first substituent whether it is alkyl group or a halogen group.
The name of the compound is 2-chlorobutane.
Interpretation:
The structural formula of
Concept Introduction:
The given compound is the branched chain alkanes. There are lots of rules are followed to write their structural formula.
Answer to Problem 65A
The structure of
Explanation of Solution
- Select the longest continuous chain containing the carbon attached to the halogen group and this is the parent chain.
- The number of the carbon atoms of the parent chain, begging from the end nearer to the first substituent whether it is alkyl group or a a halogen group.
Interpretation:
The structural formula of
Concept Introduction:
The given compound is the branched chain alkanes. Various rules are followed to write their IUPAC names.
Answer to Problem 65A
The systematic name of
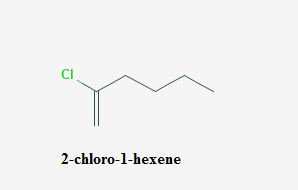
Explanation of Solution
- Select the longest continuous chain containing the carbon attached to the halogen group and this is the parent chain.
- The number of the carbon atoms of the parent chain, begging from the end nearer to the first substituent whether it is alkyl group or a halogen group.
d) Label subpart
Interpretation:
The structural formula of
Concept Introduction:
The given compound is the branched chain alkanes. Various rules are followed to write their IUPAC names.
d) Label subpart
Answer to Problem 65A
The structure of
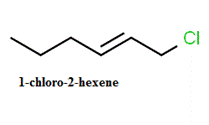
Explanation of Solution
- Select the longest continuous chain containing the carbon attached the halogen group and this is the parent chain.
- The number of the carbon atoms of the parent chain, begging from the end nearer to the first substituent whether it is alkyl group or a halogen group.
e) Label subpart
Interpretation:
The structural formula of
Concept Introduction:
The given compound is the branched chain alkanes. Various rules are followed to write their IUPAC names.
e) Label subpart
Answer to Problem 65A
The structure of
Explanation of Solution
- Select the longest continuous chain containing the carbon attached to the halogen group and this is the parent chain.
- The number of the carbon atoms of the parent chain, begging from the end nearer to the first substituent whether it is alkyl group or a halogen group.
- If more than one substituent’s are present on the parent chain, these are named in alphabetical order along with their appropriate positions.
Chapter 20 Solutions
World of Chemistry, 3rd edition
- Hi I need help on my practice final, If you could explain how to solve it, offer strategies, and dumb it down that would be amazing.arrow_forward-1 2 3 4 5 7 8 At a certain temperature this reaction follows first-order kinetics with a rate constant of 0.0635 s 2C1,0, (g) →2C1, (g)+50, (g) Suppose a vessel contains C1,0, at a concentration of 1.03 M. Calculate how long it takes for the concentration of C1,0, to decrease by 86.0%. You may assume no other reaction is important. Round your answer to 2 significant digits. e х th Earrow_forwardASAP....arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





