
(a)
Interpretation:
The missing molecule in followingsubstitution reactionhas to be indicated:
Concept Introduction:
Substitution reactionis defined as one type of
(a)
Answer to Problem 24A
The missing molecule in the given substitution reaction is CH3Cl.

Explanation of Solution
When a hydrogen atom in an
When methane is treated with chlorine molecule, one hydrogen of methane is replaced by chlorine atom and this occurs in presence of light. The reaction occurs via free radical mechanism. Light converts chlorine molecule into chlorine radical into chain initiation step which then combines with methane and convert it into methyl chloride.

(b)
Interpretation:
The missing molecule in following substitution reaction has to be indicated:
Concept Introduction:
Substitution reaction is defined as one type of chemical reaction when one atomor one group in a compound is replaced by another atom or group. When a hydrogen atom in an alkaneis replaced by a halogen atom (Cl, Br, I)is called halogenation.
(b)
Answer to Problem 24A
The missing molecule in the given substitution reaction is HCl.

Explanation of Solution
When a hydrogen atom in an alkane is replaced by a halogen atom (Cl, Br, I) is called halogenation.
When dichloromethane is treated with chlorine molecule, one hydrogen of dichloromethane is replaced by chlorine atom and this occurs in presence of light. The reaction occurs via free radical mechanism. Light converts chlorine molecule into chlorine radical into chain initiation step which then combines with dichloromethane and convert it into trichloromethane.

(c)
Interpretation:
The missing molecule in following substitution reaction has to be indicated:
Concept Introduction:
Substitution reaction is defined as one type of chemical reaction when one atomor one group in a compound is replaced by another atom or group. When a hydrogen atom in an alkaneis replaced by a halogen atom (Cl, Br, I)is called halogenation.
(c)
Answer to Problem 24A
The missing molecule in the given substitution reaction is CHCl3.
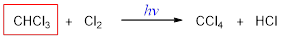
Explanation of Solution
When a hydrogen atom in an alkane is replaced by a halogen atom (Cl, Br, I) is called halogenation.
When trichloromethane is treated with chlorine molecule, one hydrogen of trichloromethane is replaced by chlorine atom and this occurs in presence of light. The reaction occurs via free radical mechanism. Light converts chlorine molecule into chlorine radical into chain initiation step which then combines with trichloromethane and convert it into tetrachloromethane.
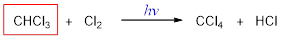
(d)
Interpretation:
The missing molecule in following substitution reaction has to be indicated:
Concept Introduction:
Substitution reaction is defined as one type of chemical reaction when one atomor one group in a compound is replaced by another atom or group. When a hydrogen atom in an alkaneis replaced by a halogen atom (Cl, Br, I)is called halogenation.
(d)
Answer to Problem 24A
The missing molecule in the given substitution reaction is Cl2.

Explanation of Solution
When a hydrogen atom in an alkane is replaced by a halogen atom (Cl, Br, I) is called halogenation.
When chloromethane is treated with chlorine molecule, one hydrogen of chloromethane is replaced by chlorine atom and this occurs in presence of light. The reaction occurs via free radical mechanism. Light converts chlorine molecule into chlorine radical into chain initiation step which then combines with chloromethane and convert it into dichloromethane.

Chapter 20 Solutions
World of Chemistry, 3rd edition
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





