
(a)
Interpretation: The water that is removed in the reaction below and the structure of ester which results need to be determined.
Concept Introduction:
Esters: Ester is formed when
(a)
Answer to Problem 4RQ
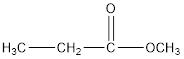
Explanation of Solution
The given reaction is,
And ester has the following general formula:
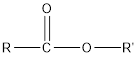
Therefore, water that is removed in the reaction below and draw the ester which results
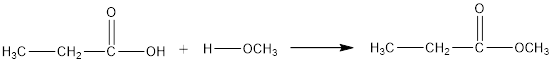
(b)
Interpretation: The name of each of the compounds in the reaction needs to be determined.
Concept Introduction: Esters: A carboxylic acid reacts with an alcohol to form an ester and a
water molecule.
(b)
Answer to Problem 4RQ
Propanoic acid, methanol and ethyl methanoate.
Explanation of Solution
In the given reaction, acid and alcohol are reacting to form an ester.
The number of C atoms in acid is 3 thus, it is a propanoic acid. The number of C atoms in alcohol is 1 thus, it is methanol. The number of C atoms formed in ester is 4 and it is formed after the removal of OH group from acid and H group from alcohol. The resultant ester will be ethyl methanoate.
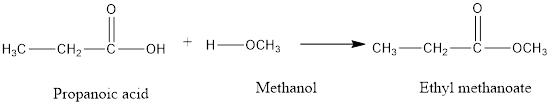
Chapter 20 Solutions
World of Chemistry, 3rd edition
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





