
Concept explainers
Some Biochemical Reactions of
Alkanes occur naturally in places other than petroleum deposits—in insects, for example. The waxy alkanes dispersed in its cuticle help protect an insect from dehydration. Some insects use volatile alkanes to defend themselves or communicate with others of the same species. Alkanes even serve as starting materials that the insect converts to other biologically important substances.
The major biosynthetic pathway leading to alkanes is by enzyme-catalysed decarboxylation (loss of
Biochemical conversion of alkanes to other substances normally begins with oxidation.
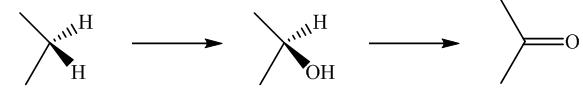
In addition to alkanes, the oxidation of drugs and other substances occurs mainly in the liver and is catalyzed by the enzyme cytochrome
Oxidation by microorganisms has been extensively studied and is often selective for certain kinds of

Tridecane
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Organic Chemistry - Standalone book
- Which reagent is needed to change an alkyne to an alkane? * Н:0+, Hg (COОH)2 N2, Pt Ог, Pd Н, Pt What reaction takes place when an alcohol is produced during the net addition of water across the double bond of an alkene? * Dehydrogenation Hydrogenation Dehydration Hydrationarrow_forwardIndicate whether each statement is true or false. Alkanes do not have any carbon-carbon multiple bonds. Cyclobutane contains a four-membered ring. Alkenes contain carbon-carbon triple bonds. Alkynes contain carbon-carbon double bonds. Pentene is a saturated hydrocarbon. 1-pentene is an unsaturated hydrocarbon. Cyclohexane is an aromatic hydrocarbon. The methyl group contains one less hydrogen atom than methane.arrow_forwardWhat is the structure of a compound with the formula C7H14O that has an ether and cyclobutane ring, including its IUPAC name? In addition, what is the structure of the compound with the formula C7H14O that does NOT contain the functional groups: epoxide, ether, cycloalkane, alcohol, alkene. Indicate the functional groups used that are present in the structure made for this compound.arrow_forward
- Indicate whether each statement is true or false. Two generic isomers of pentane are n-pentane and neo-pentane. Alkenes can have cis and trans isomers around the CC double bond. Alkynes can have cis and trans isomers around CC triple bond.arrow_forward16. An atom or group of atoms that can give organic compounds distinct chemical and physical properties. 21. When a compound with the general formula R-COOH loses a proton, the product that remains is described with this term. Its general formula is R-COO- 24. A class of organic compounds in which three or more carbons form a ring structure. All of the carbon-to-carbon bonds are single bonds in this family of compounds.arrow_forwardComplete the equations by choosing the right products in preparation and chemical properties of alkanes. 1. Propane + HOSO3H 2. Butane + HONO2 3. Octane + HONO2 4. CH3CH2CBr3 + Br2 5. C4H8Br2 + Br2arrow_forward
- Select the correct statements about the substitution and elimination reactions of haloalkanes. * A- Substitution occurs at the ?-carbon atom because it has the lowest electron density. B- During elimination, a hydrogen is removed from the ꞵ-carbon because this is the position with the highest acidity in the haloalkane. C- All haloalkanes can undergo at least one elimination reaction. D- Elimination only occurs when the base is very strong. E- Substitution only occurs when the nucleophile is weakly basic.arrow_forwardExplain why alkenes are much more reactive than alkanes towards chlorine (CI2) or bromine (Br2) in the dark at room temperature, and why alkanes do not react with HCI (g) or HBr (g) whereas alkenes do.arrow_forwardWhy does Halogenation increase the reactivity of Alkanes?arrow_forward
- Name and draw structural formulas for all alkenes with the molecular formula C5H10. As you draw these alkenes, remember that cis and trans isomers are different compounds and must be counted separatelyarrow_forwardThe following is a structural diagram for penicillin G, an antibiotic compound with outstanding antibacterial activity. It is obtained from the liquid filtrate of molds. HO H H H C-C N- H Penicillin G can be described as an Organic Co -N- organic inorganic CH₂ H CH₂ -COOH compound. One functional group found in penicillin G is the ◆ grouparrow_forwardThe number of secondary hydrogen(s) in this compound. CH- CHCH 4 This alkyne has been identified as a component of the hydrocarbon-rich atmospheres of Uranus, Neptune, and Pluto. O 1,3-pentadiyne O 1,3-butadiyne O 1,3-hexadiyne O 1,3-heptadiyne The Functional group of aromatic hydrocarbons. OC-C- single bond O-C-C- double bond OC-C- triple bond benzene ringarrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning  Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning




