
Concept explainers
(a)
Interpretation:
Bond angle at central atom for
Concept Introduction:
Valence Shell Electron Pair Repulsion model predicts shape by inclusion of bond angles and most distant arrangement of atoms that leads to minimum repulsion. For the molecules that have no lone pairs around the central atom the bonded-atom unshared -pair arrangement is decided by the table as follows:
In order to determine the shape the steps to be followed are indicated as follows:
- 1. Lewis structure of molecule should be written.
- 2. The type electron arrangement around the central atom should be identified around the central atom. This essentially refers to determination of bond pairs and unshared or lone pairs around central atoms.
- 3. Then bonded-atom unshared -pair arrangement that can maximize the distance of electron pairs about central atom determines the shape.
For molecules that have lone pairs around central atom, lone pairs influence shape, because there are no atoms at the positions occupied by these lone pairs. The key rule that governs the molecular shape, in this case, is the extent of lone –lone pair repulsions are far greater than lone bond pair or bond pair-bond pair repulsions. The table that summarized the molecular shapes possible for various combinations of bonded and lone pairs are given as follows:
(a)
Answer to Problem 2E.17E
In
Explanation of Solution
Total valence electrons are sum of the valence electrons on each atom in
The skeleton structure in
These 7 electron pairs are allotted as lone pairs to satisfy respective octets. Hence, the Lewis structure in
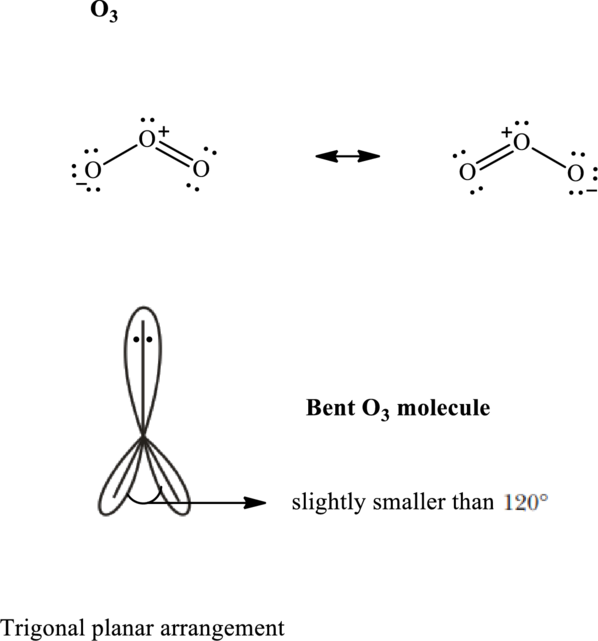
It is evident that
(b)
Interpretation:
Bond angle at central atom for
Concept Introduction:
Refer to part (a).
(b)
Answer to Problem 2E.17E
In azide ion, bond angle is
Explanation of Solution
Total valence electrons are sum of the valence electrons on each atom in
The skeleton structure in
These 6 electron pairs are allotted multiple bonds to satisfy respective octets. Hence, the Lewis structure in
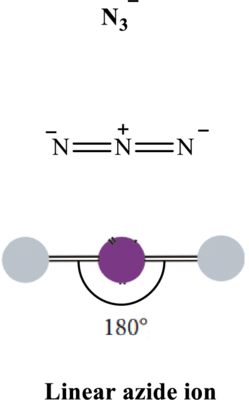
It is evident that
(c)
Interpretation:
Bond angle at central atom for
Concept Introduction:
Refer to part (a).
(c)
Answer to Problem 2E.17E
In
Explanation of Solution
Thus total valence electrons is sum of the valence electrons on each atom along with charge in
The skeleton structure in
These 6 electron pairs are allotted multiple bonds to satisfy respective octets. Hence, the Lewis structure in
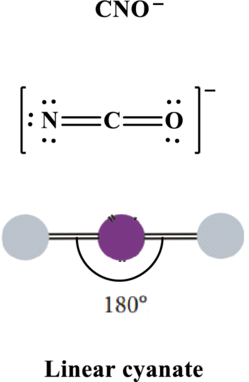
It is evident that
(d)
Interpretation:
Bond angle at central atom for
Concept Introduction:
Refer to part (a).
(d)
Answer to Problem 2E.17E
Bond angle in
Explanation of Solution
Total valence electrons are sum of the valence electrons on atom in
The skeleton structure in
This 1 electron pair is allotted as lone pair. Hence, the Lewis structure in
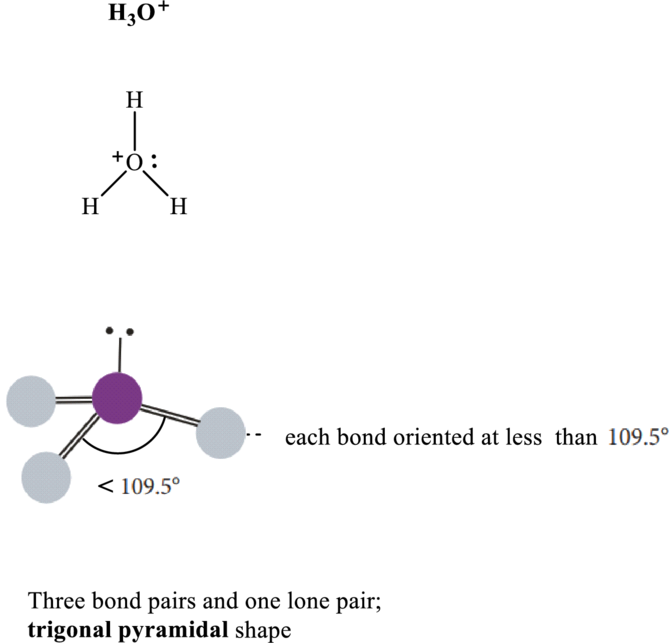
It is evident that in
Want to see more full solutions like this?
Chapter 2 Solutions
Chemical Principles: The Quest for Insight
- It is possible to write a simple Lewis structure for the SO42- ion, involving only single bonds, which follows the octet rule. However, Linus Pauling and others have suggested an alternative structure, involving double bonds, in which the sulfur atom is surrounded by six electron pairs. (a) Draw the two Lewis structures. (b) What geometries are predicted for the two structures? (c) What is the hybridization of sulfur in each case? (d) What are the formal charges of the atoms in the two structures?arrow_forwardFrom their Lewis structures, determine the number of sand p bonds in each of the following molecules or ions:(a) CO2; (b) cyanogen, 1CN22; (c) formaldehyde, H2CO;(d) formic acid, HCOOH, which has one H and two O atomsattached to C.arrow_forward4. (a) Draw the shape of the atomic valence orbitals formed by the overlaping of two fluoride 2p atomic orbitals. (b) Draw the molecular orbital diagrams for F2 and F2*. Identify their bond order and magnetic properties. (c) An unstable nucleus exhibit radioactivity. (i) Explain how the number of protons and neutrons in a radioactive nucleus can be used to predict its probable mode decay. (ii) Illustrate your answer in (i) with a schematic graph.arrow_forward
- . Assume that the third-period element phosphorus forms a diatomic molecule, P2, in an analogous way as nitrogen does to form N2. (a) Write the electronic configuration for P2. Use [Ne2] to represent the electron configuration for the first two periods. (b) Calculate its bond order. (c) What are its magnetic properties (diamagnetic or paramagnetic)?arrow_forward2.arrow_forwardLike several other bonds, carbon-oxygen bonds havelengths and strengths that depend on the bond order. Draw Lewis structures for the following species, and arrange them in order of increasing carbon-oxygen bond length and then by increasing carbon-oxygen bond strength: (a) CO; (b) CO₃²⁻; (c) H₂CO;(d) CH₄O; (e) HCO₃(H attached to O).arrow_forward
- Draw the Lewis structure with lowest formal charges, and determine the charge of each atom in (a) OCS; (b) NO. (C)CN−; (d) ClO−.arrow_forwardThe sulfate ion can be represented with four S-O bonds or with two S-O and two So=O bonds.(a) Which representation is better from the standpoint of formal charges?(b) What is the shape of the sulfate ion, and what hybrid orbitals of S are postulated for the σ bonding?(c) In view of the answer to part (b), what orbitals of S must be used for the π bonds? What orbitals of O?(d) Draw a diagram to show how one atomic orbital from S and one from O overlap to form a π bond.arrow_forwardThe Lewis structure of BH2Cl (a) Is the molecule polar or nonpolar? (b) What is the hybridization of the carbon atom? (c) What is the geometric shape of the molecule?arrow_forward
- Chemical species are said to be isoelectronic if they have the same Lewis structure (regardless of charge). Consider these ions and write a Lewis structure for a neutral molecule that is isoelectronic with them. (a) CN–, (b) NH4+ (c) CO3 2–arrow_forward(a) Find the angle u between adjacent nearest-neighbor bonds in the silicon lattice. Recall that each silicon atom is bonded to four of its nearest neighbors.The four neighbors form a regular tetrahedron— a pyramid whose sides and base are equilateral triangles. (b) Find the bond length, given that the atoms at the corners of the tetrahedron are 388 pm apart.arrow_forward2(a) Provide the Lewis structures for both CH3OH and C2H3Cl. 2(b) What is the largest bond angle among all the bond angles in CH3OH and C2H3Cl? Listthe three atoms making this largest bond angle, and estimate the value of the angle.2(c) What intermolecular forces are present(i) between CH3OH molecules?(ii) between C2H3Cl molecules?arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning


