
Concept explainers
(a)
Interpretation:
The bond length of
Concept Introduction:
The bond length is estimated to be average of covalent radii of two atoms within a bond. Each ion contributes to the bond length as illustrated as follows:
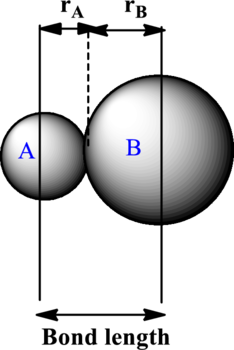
The expression to calculate the bond length is as follows:
Here,
(b)
Interpretation:
The bond length of
Concept Introduction:
Refer to part (a).
(c)
Interpretation:
The bond length of nitrogen- nitrogen triple bond in
Concept Introduction:
Refer to part (a).
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Chemical Principles: The Quest for Insight
- Chemical species are said to be isoelectronic if they have the same Lewis structure (regardless of charge). Consider these ions and write a Lewis structure for a neutral molecule that is isoelectronic with them. (a) CN–, (b) NH4+ (c) CO3 2–arrow_forward(a) How does a polar molecule differ from a nonpolar one? (b) Atoms X and Y have different electronegativities. Will the diatomic molecule X—Y necessarily be polar? Explain. (c) What factors affect the size of the dipole moment of a diatomic molecule?arrow_forward. Assume that the third-period element phosphorus forms a diatomic molecule, P2, in an analogous way as nitrogen does to form N2. (a) Write the electronic configuration for P2. Use [Ne2] to represent the electron configuration for the first two periods. (b) Calculate its bond order. (c) What are its magnetic properties (diamagnetic or paramagnetic)?arrow_forward
- Like several other bonds, carbon-oxygen bonds havelengths and strengths that depend on the bond order. Draw Lewis structures for the following species, and arrange them in order of increasing carbon-oxygen bond length and then by increasing carbon-oxygen bond strength: (a) CO; (b) CO₃²⁻; (c) H₂CO;(d) CH₄O; (e) HCO₃(H attached to O).arrow_forward(a) Find the angle u between adjacent nearest-neighbor bonds in the silicon lattice. Recall that each silicon atom is bonded to four of its nearest neighbors.The four neighbors form a regular tetrahedron— a pyramid whose sides and base are equilateral triangles. (b) Find the bond length, given that the atoms at the corners of the tetrahedron are 388 pm apart.arrow_forwardMixing SbCl3 and GaCl3 in a 1:1 molar ratio using liquid sulfur dioxide as a solvent gives a solidionic compound with the empirical formula GaSbCl6. A controversy arose over whether this compoundis [SbCl2]+[GaCl4]− or [GaCl2]+[SbCl4]−.(a) Predict the molecular structure of the two anions from the two choices using VSEPR theory.(b) It is learned that the cation in the compound has a bent structure. Based on this fact, whichformulation is the correct one?arrow_forward
- Common exceptions to the octet rule are compounds and polyatomic ions with central atoms having more than 8 electrons around them. Phosphorus pentafluoride, PF5; sulfur tetrafluoride, SF4; xenon tetrafluoride, XeF4; and tri-iodide ion, I3, are all examples of exceptions to the octet rule. (a) Draw the Lewis structures of these substances.(b) For which elements in these substances can theatoms have more than 8 electrons around them?(c) How can the atoms of the elements youidentified in Part (b) be surrounded by morethan 8 electrons?arrow_forwardWhich substance in each of the following pairs would you expect to have the higher boiling point? (a) Ne or Xe, (b) CO2 or CS2, (c) CH4 or Cl2, (d) F2 or LiF, (e) NH3 or PH3 (a) Ne; (b) CS2; (c) CH4; (d) F2 ; (e) NH3 (a) Xe; (b) CS2; (c) Cl2; (d) LiF ; (e) PH3 O (a) Xe; (b) CS2; (c) Cl2; (d) LiF ; (e) NH3 (a) Xe; (b) CS2; (c) Cl2; (d) F2 ; (e) NH3 (a) Xe; (b) C02; (c) CH4 ; (d) LiF ; (e) PH3arrow_forwardDraw the Lewis structure with lowest formal charges, and determine the charge of each atom in (a) OCS; (b) NO. (C)CN−; (d) ClO−.arrow_forward
- Nitrogen trifluoride (NF3) is used in the electronics industry to clean surfaces. NF3 is also a potent greenhouse gas. (A) Draw the Lewis structure of NF3 and determine its molecular geometry. (B) BF3 and NF3 both have three covalently bonded fluorine atoms around a central atom. Do they have the same dipole moment? (C) Could BF3 also behave as a greenhouse gas? Explain why or why not.arrow_forwardIn addition to ammonia, nitrogen forms three other hydrides: hydrazine (N2H4), diazene (N2H2), and tetrazene (N4H4).(a) Use Lewis structures to compare the strength, length, and order of the nitrogen-nitrogen bonds in hydrazine, diazene, and N2.(b) Tetrazene (atom sequence H2NNNNH2) decomposes above 08C to hydrazine and nitrogen gas. Draw a Lewis structure for tetrazene, and calculate ΔH°rxn for this decomposition.arrow_forwardAn important starting material for the manufacture ofpolyphosphazenes is the cyclic molecule (NPCl₂)₃. The mol-ecule has a symmetrical six-membered ring of alternating N and P atoms, with the Cl atoms bonded to the P atoms. The nitrogen-phosphorus bond length is significantly less than that expectedfor an N−P single bond.(a) Draw a likely Lewis structure for the molecule.(b) How many lone pairs of electrons do the ring atoms have?(c) What is the order of the nitrogen-phosphorus bond?arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
