
Concept explainers
Interpretation:
The other name for secondary amides has to be chosen from the given options.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Organic compounds contain heteroatom also. Some of them are nitrogen, sulfur, oxygen etc. Nitrogen containing organic compounds are of two important types and they are
One of the
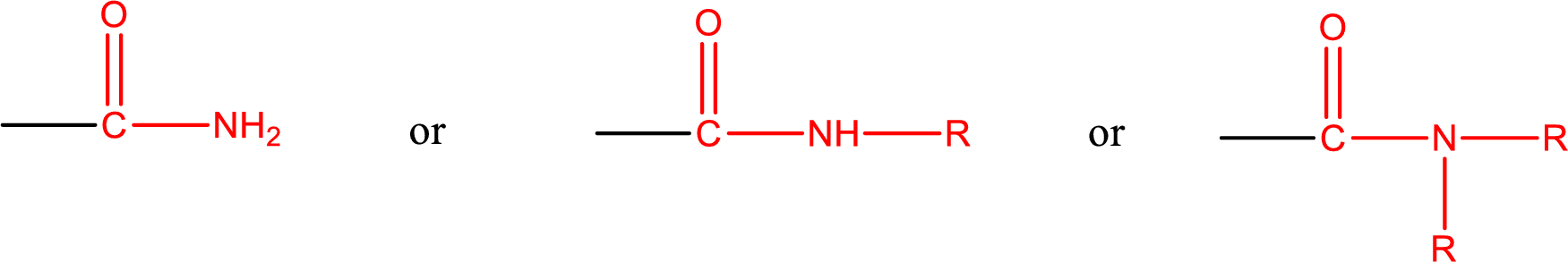
Amides are also classified as primary, secondary, and tertiary amide.
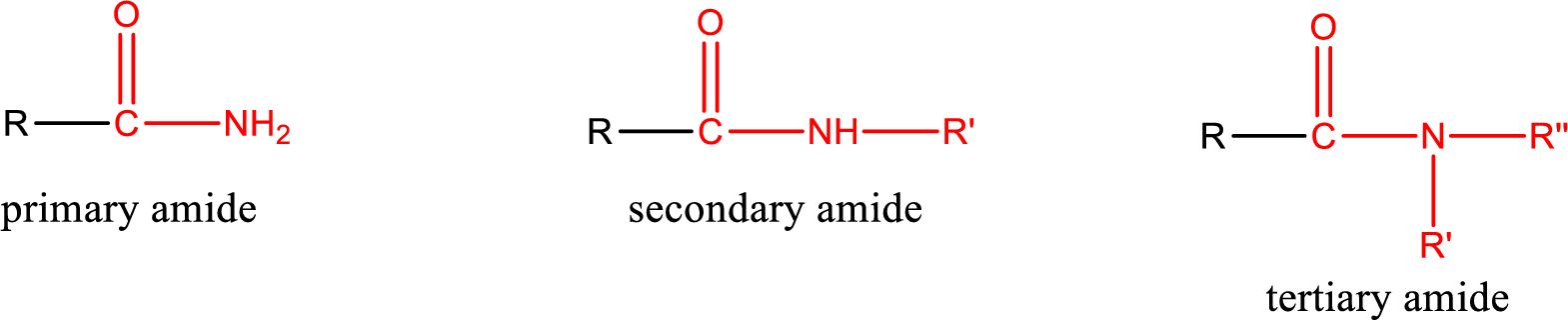
Primary amide is the one that has a nitrogen atom that is bonded to two hydrogen atoms. Primary amides are also known as unsubstituted amides.
Secondary amide is the one that has a nitrogen atom that is bonded to one hydrogen atom and one alkyl (or aryl) group. Secondary amides are also known as monosubstituted amides.
Tertiary amide is the one that has a nitrogen atom that is bonded to two alkyl (or aryl) groups. Tertiary amides are also known as disubstituted amides.
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
General, Organic, and Biological Chemistry
- → BINDERIYA GANBO... BINDERIYA GANBO. AP Biology Notes Gamino acid chart - G... 36:22 司 10 ☐ Mark for Review Q 1 Hide 80 8 2 =HA O=A¯ = H₂O Acid HIO HBrO HCIO Question 10 of 35 ^ Σ DELL □ 3 % Λ & 6 7 * ∞ 8 do 5 $ 4 # m 3 ° ( 9 Highlights & Notes AXC Sign out Carrow_forwardWhich representation(s) show polymer structures that are likely to result in rigid, hard materials and those that are likely to result in flexible, stretchable, soft materials?arrow_forward3. Enter the molecular weight of the product obtained from the Williamson Ether Synthesis? OH OH & OH excess CH3l Ag₂Oarrow_forward
- Please answer 1, 2 and 3 on the endarrow_forwardIn the box below, specify which of the given compounds are very soluble in polar aprotic solvents. You may select more than one compound. Choose one or more: NaCl NH4Cl CH3CH2CH2CH2CH2CN CH3CH2OH hexan-2-one NaOH CH3SCH3arrow_forwardOn the following structure, select all of the atoms that could ACCEPT a hydrogen bond. Ignore possible complications of aromaticity. When selecting be sure to click on the center of the atom.arrow_forward
- Rank the compounds below from lowest to highest melting point.arrow_forward18 Question (1 point) Draw the line structure form of the given partially condensed structure in the box provided. :ÖH HC HC H2 ΙΩ Н2 CH2 CH3 CH3 partially condensed formarrow_forwardsomeone else has already submitted the same question on here and it was the incorrect answer.arrow_forward
- The reaction: 2NO2(g) ⇌ N2O4(g) is an exothermic reaction, ΔH=-58.0 kJ/molrxn at 0°C the KP is 58.If the initial partial pressures of both NO2(g) and N2O4(g) are 2.00 atm:A) Is the reaction at equilibrium? If not, what is the value of Q? B) Which direction will the reaction go to reach equilibrium? C) Use an ICE table to find the equilibrium pressures.arrow_forwardThe dissociation of the weak acid, nitrous acid, HNO2, takes place according to the reaction: HNO2 (aq) ⇌ H+(aq) + NO2–(aq) K=7.2 X 10-4 When 1.00 mole of HNO2 is added to 1.00 L of water, the H+ concentration at equilibrium is 0.0265 M.A) Calculate the value of Q if 1.00 L of water is added? B) How will reaction shift if 1.00 L of water is added?arrow_forwardSuppose a certain copolymer elastomeric material “styrene-butadiene rubber”) contains styrene ("S") monomers –(C8H8)– and butadiene ("B") monomers –(C4H6)– and that their numerical ratio S:B = 1:8. What is the mass ratio mS:mB of the two monomers in the material? What is the molecular mass M of a macromolecule of this copolymer with degree of polymerization n = 60,000? Data: AC = 12.01 u, AH = 1.008 u.arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,

