
(a)
Interpretation:
To propose a mechanism for the formation of succinic anhydride in the presence of acetic anhydride and how acetic anhydride makes it easier to form the succinic anhydride.
Concept introduction:
Succinic anhydride is a type of cyclic anhydride formed by the dehydration of dicarboxylic acid that is succinic acid. The cyclic anhydride is formed easily in the presence of anhydride such as acetic anhydride.
The reaction is:
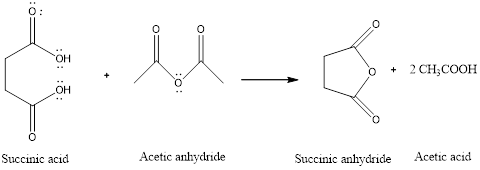
(b)
Interpretation:
To propose a mechanism for the formation of succinic anhydride in the presence of acetic anhydride and how acetic anhydride makes it easier to form the succinic anhydride.
Concept introduction:
Succinic anhydride is a type of cyclic anhydride formed by the dehydration of dicarboxylic acid that is succinic acid. The cyclic anhydride is formed easily in the presence of anhydride such as acetic anhydride.
The reaction is:

Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Organic Chemistry (8th Edition)
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardThe Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forward
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardOnly 100% sure experts solve it correct complete solutions need to get full marks it's my quiz okkkk.take your time but solve full accurate okkk chemistry expert solve itarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forward

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


