
Life: The Science of Biology
11th Edition
ISBN: 9781319010164
Author: David E. Sadava, David M. Hillis, H. Craig Heller, Sally D. Hacker
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 13.2, Problem 3R
Summary Introduction
To review: The interaction between proteins and DNA.
Introduction:
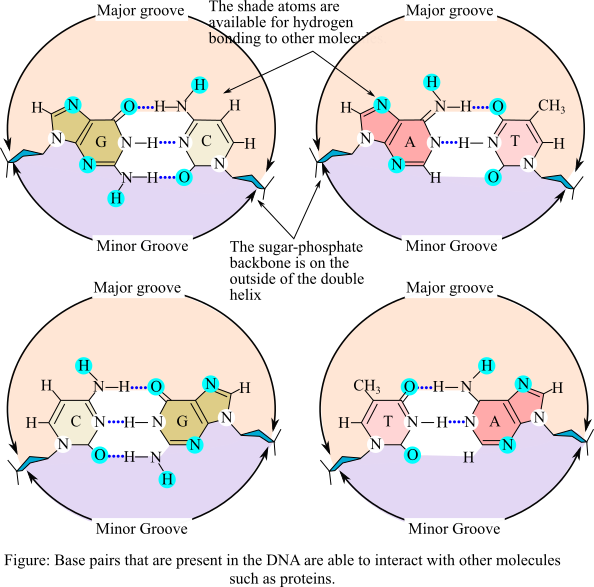
DNA stands for deoxyribonucleic acid and it is known as the genetic material for all the organisms. Proteins play a crucial role in regulating the central dogma, mainly the transcription process of DNA to RNA. It is involved in replication, repair, and packaging process. The proteins and DNA interact during this process and some of them occur in the major groove where the bases are exposed and some occur in the minor groove, which comprises carbohydrate sections of DNA. The structure of the DNA with major and minor grooves are shown below:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Consider the following in light of the concept of levels of structure (primary, secondary, tertiary, quaternary)as defined for proteins.(a) What level is shown by double-stranded DNA?(b) What level is shown by tRNA?(c) What level is shown by mRNA?
To create a DNA:RNA hybrid from a short stretch of DNA with the sequence
5'-GGCTAAGTATGCCTAGTAGC-3', design the corresponding RNA
sequence. Indicate the sequence in a 5' to 3' manner. What type of helix (A, B
or Z) will this double-stranded nucleic acid form?
Would you expect the double helix in a short segment of DNA to be more stable in a storage solution of sodium phosphate
buffer at pH 7.0 or in pure water? Why?
pure water; because any cations in the storage solution would prevent complementary bases from forming
ionic interactions
sodium phosphate buffer at pH 7.0; because a neutral pH is required to maintain the covalent bonds between
complementary bases
O pure water; because the high dielectric constant of water is sufficient to stabilize the covalent bonds between
deoxyriboses in the DNA backbone
sodium phosphate buffer at pH 7.0; because the presence of water and sodium neutralizes the charge of phosphate
groups in the DNA backbone
Chapter 13 Solutions
Life: The Science of Biology
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- (a) Draw diagrams to show how the four synthetic oligonucleotides below could base-pair to form a stable model Holliday junction. W 5' GATCGCATTGTAGCCGTAGGTCCACTGTAA 3’ X 5' GTCCCATACGTAGCCGTAGGACATGTACCG 3' Y 5' CGGTACATGTCCTACGGCTACAATGCGATC 3' Z 5' TTACAGTGGACCTACGGCTACGTATGGGAC 3' I and 21arrow_forwardDescribe the functions of the following proteins during DNA breaks and repair: (i) Ku70 (ii) Uracil DNA glycosylasearrow_forwardThe hydrophobic effect explains why: O Water regulates pH in the interstitial fluid between eukaryotic cells. O Salt bridges form between oppositely charged histones and DNA in an aqueous environment. Nitrogenous bases are in the interior of the helix, when DNA is in an aqueous environment. Oil forms a homogenous mixture with polar solvents, like acetone.arrow_forward
- Given the following eukaryotic DNA strand, transcribe and translate the DNA into a polypeptide using the 3’ – 5’ strand as the template. You may use drawings, diagrams, colours and annotations to describe how the DNA strand will be synthesized into a functional protein. (32) (KEY: The letters SBMD are “made up” nucleic acids that depict non-coding regions in the DNA, hypothetically S pairs with B and M pairs with D). 5’ - TATAAAAASSMSBMDATGSBDCCMBDBAATBSMDSTGTGTCCTMSBAG – 3’arrow_forwardIf a DNA-binding protein “reads” a short stretch of DNA and detects the following “second” genetic code provided by the functional groups located on each base as H-HD-CH3-HA-HA-HA-HA-HD, then what is the corresponding sequence of bases? (H = hydrogen, HD = hydrogen donor, HA = hydrogen acceptor, CH3 = methyl)arrow_forwardIn what ways are the structures of an α helix in a protein and thedouble helix of DNA similar, and in what ways are theydifferent?arrow_forward
- What are the complementary base pairs in DNA-RNA interactions? Answer format: Base 1(one letter symbol)-Base 2 (one letter symbol, or B-B*(hypothetical N-base) In the lengthening of a polynucleotide chain, which type of nucleotide subunit (name please not the formula) would bond to its 3’ end? How many 3’,5’-phosphodiester linkages are present in a tetranucleotide segment of a nucleic acid?arrow_forwardHelicases are motor proteins that translocate on nucleic acids: Describe the structure of the conserved RecA fold of helicasesarrow_forwardGiven the following eukaryotic DNA strand, transcribe and translate the DNA into a polypeptide using the 3’ – 5’ strand as the template. use drawings, diagrams, colours and annotations to describe how the DNA strand will be synthesized into a functional protein. 5’ - TATAAAAASSMSBMDATGSBDCCMBDBAATBSMDSTGTGTCCTMSBAG – 3’ (KEY: The letters SBMD are “made up” nucleic acids that depict non-coding regions in the DNA, hypothetically S pairs with B and M pairs with D).arrow_forward
- Select TRUE or FALSE for each of the following statements: 1. Only one of the three phosphate groups present in each nucleotide precursor remains present in a DNA polymer. 2. Starch and cellulose are alike in that both contain sugars bonded together in identical ways. 3. The coding strand of DNA is complementary in sequence to the corresponding MRNA. 4. Ribosomal RNA (rRNA) is synthesised by ribosomes in the process of translation. 5. Polyribosomes speed up the rate of transcription.arrow_forward2) When DNA is placed in distilled water, which is pH 7.0, it denatures (i.e., the two strands separate). The pH inside a cell is generally 7.2-7.5, depending on the organism, but DNA is generally double-stranded under physiological conditions. Briefly explain, in your own words, why DNA denatures when placed in distilled water but not when it is inside a cell. [Reminder: the pKa for the phosphate groups in the sugar-phosphate backbone of a strand of DNA is 2.14]arrow_forwardWhich of the molecules of RNA is the most likely to fold into a specific structure as a result of intramolecular (within itself) base-pairing? Explain. 5′-CCCUAAAAAAAAAAAAAAAAUUUUUUUUUUUUUUUUAGGG-3′ 5′-UGUGUGUGUGUGUGUGUGUGUGUGUGUGUGUGUGUGUGUG-3′ 5′-AAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAA-3′ 5′-GGAAAAGGAGAUGGGCAAGGGGAAAAGGAGAUGGGCAAGG-3′arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education
Macromolecules | Classes and Functions; Author: 2 Minute Classroom;https://www.youtube.com/watch?v=V5hhrDFo8Vk;License: Standard youtube license