
General, Organic, and Biological Chemistry
7th Edition
ISBN: 9781285853918
Author: H. Stephen Stoker
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.19EP
Convert the expanded structural formulas in Problem 12-15 into skeletal structural formulas.
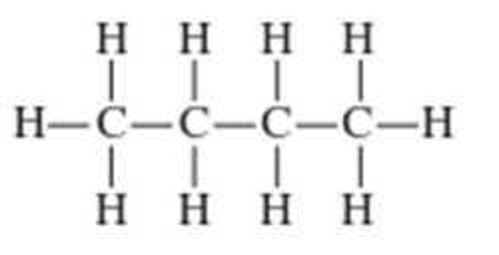
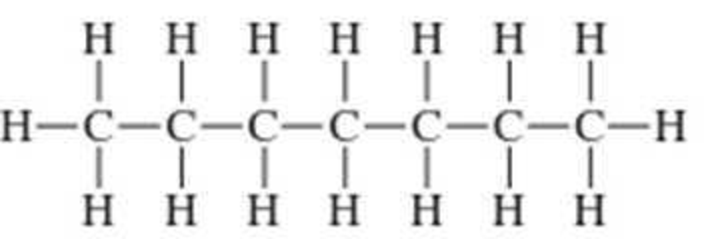
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Find a molecular formula for these unknowns
(ME EX2) Prblms 8-11 Can you please explain problems 8 -11 to me in detail, step by step? Thank you so much! If needed color code them for me.
Don't used hand raiting
Chapter 12 Solutions
General, Organic, and Biological Chemistry
Ch. 12.1 - Prob. 1QQCh. 12.1 - Prob. 2QQCh. 12.2 - Prob. 1QQCh. 12.2 - Prob. 2QQCh. 12.3 - Prob. 1QQCh. 12.3 - Prob. 2QQCh. 12.4 - Prob. 1QQCh. 12.4 - Prob. 2QQCh. 12.4 - Prob. 3QQCh. 12.5 - Prob. 1QQ
Ch. 12.5 - Prob. 2QQCh. 12.5 - Prob. 3QQCh. 12.6 - Prob. 1QQCh. 12.6 - Prob. 2QQCh. 12.6 - Prob. 3QQCh. 12.6 - Prob. 4QQCh. 12.7 - Prob. 1QQCh. 12.7 - Prob. 2QQCh. 12.8 - Prob. 1QQCh. 12.8 - Prob. 2QQCh. 12.8 - Prob. 3QQCh. 12.8 - Prob. 4QQCh. 12.8 - Prob. 5QQCh. 12.8 - Prob. 6QQCh. 12.8 - Prob. 7QQCh. 12.9 - Prob. 1QQCh. 12.9 - Prob. 2QQCh. 12.10 - Prob. 1QQCh. 12.10 - Prob. 2QQCh. 12.11 - Prob. 1QQCh. 12.11 - Prob. 2QQCh. 12.11 - Prob. 3QQCh. 12.12 - Prob. 1QQCh. 12.12 - Prob. 2QQCh. 12.12 - Prob. 3QQCh. 12.13 - Prob. 1QQCh. 12.13 - Prob. 2QQCh. 12.13 - Prob. 3QQCh. 12.14 - Prob. 1QQCh. 12.14 - Prob. 2QQCh. 12.14 - Prob. 3QQCh. 12.15 - Prob. 1QQCh. 12.15 - Prob. 2QQCh. 12.16 - Prob. 1QQCh. 12.16 - Prob. 2QQCh. 12.16 - Prob. 3QQCh. 12.17 - Prob. 1QQCh. 12.17 - Prob. 2QQCh. 12.17 - Prob. 3QQCh. 12.17 - Prob. 4QQCh. 12.18 - Prob. 1QQCh. 12.18 - Prob. 2QQCh. 12.18 - Prob. 3QQCh. 12.18 - Prob. 4QQCh. 12 - Prob. 12.1EPCh. 12 - Prob. 12.2EPCh. 12 - Prob. 12.3EPCh. 12 - Prob. 12.4EPCh. 12 - Indicate whether each of the following situations...Ch. 12 - Indicate whether each of the following situations...Ch. 12 - Prob. 12.7EPCh. 12 - Prob. 12.8EPCh. 12 - What is the difference between a saturated...Ch. 12 - Prob. 12.10EPCh. 12 - Prob. 12.11EPCh. 12 - Prob. 12.12EPCh. 12 - Prob. 12.13EPCh. 12 - Prob. 12.14EPCh. 12 - Prob. 12.15EPCh. 12 - Prob. 12.16EPCh. 12 - Prob. 12.17EPCh. 12 - Prob. 12.18EPCh. 12 - Convert the expanded structural formulas in...Ch. 12 - Prob. 12.20EPCh. 12 - Prob. 12.21EPCh. 12 - Prob. 12.22EPCh. 12 - Prob. 12.23EPCh. 12 - Prob. 12.24EPCh. 12 - Prob. 12.25EPCh. 12 - Prob. 12.26EPCh. 12 - Indicate whether each of the following would be...Ch. 12 - Indicate whether each of the following would be...Ch. 12 - Prob. 12.29EPCh. 12 - Prob. 12.30EPCh. 12 - Prob. 12.31EPCh. 12 - Prob. 12.32EPCh. 12 - Prob. 12.33EPCh. 12 - How many of the numerous seven-carbon alkane...Ch. 12 - Prob. 12.35EPCh. 12 - For each of the following pairs of structures,...Ch. 12 - Prob. 12.37EPCh. 12 - Prob. 12.38EPCh. 12 - Prob. 12.39EPCh. 12 - Prob. 12.40EPCh. 12 - Prob. 12.41EPCh. 12 - What is the name of the IUPAC prefix associated...Ch. 12 - What is the IUPAC name for each of the following...Ch. 12 - What is the IUPAC name for each of the following...Ch. 12 - Prob. 12.45EPCh. 12 - What is the chemical formula for each of the...Ch. 12 - Prob. 12.47EPCh. 12 - Prob. 12.48EPCh. 12 - Prob. 12.49EPCh. 12 - Prob. 12.50EPCh. 12 - Prob. 12.51EPCh. 12 - Prob. 12.52EPCh. 12 - Draw a condensed structural formula for each of...Ch. 12 - Draw a condensed structural formula for each of...Ch. 12 - Prob. 12.55EPCh. 12 - Prob. 12.56EPCh. 12 - Explain why the name given for each of the...Ch. 12 - Prob. 12.58EPCh. 12 - Indicate whether or not the two alkanes in each of...Ch. 12 - Prob. 12.60EPCh. 12 - How many of the 18 C8 alkane constitutional...Ch. 12 - Prob. 12.62EPCh. 12 - Prob. 12.63EPCh. 12 - Prob. 12.64EPCh. 12 - Prob. 12.65EPCh. 12 - Prob. 12.66EPCh. 12 - Do the line-angle structural formulas in each of...Ch. 12 - Do the line-angle structural formulas in each of...Ch. 12 - Convert each of the condensed structural formulas...Ch. 12 - Convert each of the condensed structural formulas...Ch. 12 - Assign an IUPAC name to each of the compounds in...Ch. 12 - Prob. 12.72EPCh. 12 - Prob. 12.73EPCh. 12 - Prob. 12.74EPCh. 12 - For each of the alkane structures in Problem...Ch. 12 - Prob. 12.76EPCh. 12 - Prob. 12.77EPCh. 12 - Prob. 12.78EPCh. 12 - Prob. 12.79EPCh. 12 - Prob. 12.80EPCh. 12 - Prob. 12.81EPCh. 12 - Prob. 12.82EPCh. 12 - Draw condensed structural formulas for the...Ch. 12 - Draw condensed structural formulas for the...Ch. 12 - To which carbon atoms in a hexane molecule can...Ch. 12 - Prob. 12.86EPCh. 12 - Prob. 12.87EPCh. 12 - Prob. 12.88EPCh. 12 - Prob. 12.89EPCh. 12 - Prob. 12.90EPCh. 12 - Prob. 12.91EPCh. 12 - Prob. 12.92EPCh. 12 - Prob. 12.93EPCh. 12 - Using the general formula for a cycloalkane,...Ch. 12 - Prob. 12.95EPCh. 12 - Prob. 12.96EPCh. 12 - Prob. 12.97EPCh. 12 - Prob. 12.98EPCh. 12 - How many secondary carbon atoms are present in...Ch. 12 - Prob. 12.100EPCh. 12 - Prob. 12.101EPCh. 12 - Assign an IUPAC name to each of the following...Ch. 12 - Prob. 12.103EPCh. 12 - What is wrong with each of the following attempts...Ch. 12 - Draw line-angle structural formulas for the...Ch. 12 - Draw line-angle structural formulas for the...Ch. 12 - Prob. 12.107EPCh. 12 - Prob. 12.108EPCh. 12 - Prob. 12.109EPCh. 12 - Prob. 12.110EPCh. 12 - Determine the number of constitutional isomers...Ch. 12 - Determine the number of constitutional isomers...Ch. 12 - Prob. 12.113EPCh. 12 - Determine whether cistrans isomerism is possible...Ch. 12 - Prob. 12.115EPCh. 12 - Prob. 12.116EPCh. 12 - Prob. 12.117EPCh. 12 - Indicate whether the members of each of the...Ch. 12 - Prob. 12.119EPCh. 12 - Prob. 12.120EPCh. 12 - Prob. 12.121EPCh. 12 - Prob. 12.122EPCh. 12 - Which member in each of the following pairs of...Ch. 12 - Prob. 12.124EPCh. 12 - Prob. 12.125EPCh. 12 - Prob. 12.126EPCh. 12 - Answer the following questions about the...Ch. 12 - Prob. 12.128EPCh. 12 - Prob. 12.129EPCh. 12 - Prob. 12.130EPCh. 12 - Prob. 12.131EPCh. 12 - Prob. 12.132EPCh. 12 - Prob. 12.133EPCh. 12 - Write structural formulas for all the possible...Ch. 12 - Assign an IUPAC name to each of the following...Ch. 12 - Assign an IUPAC name to each of the following...Ch. 12 - Prob. 12.137EPCh. 12 - Prob. 12.138EPCh. 12 - Prob. 12.139EPCh. 12 - Draw structural formulas for the following...Ch. 12 - Prob. 12.141EPCh. 12 - Prob. 12.142EPCh. 12 - Prob. 12.143EPCh. 12 - Prob. 12.144EPCh. 12 - Prob. 12.145EPCh. 12 - Prob. 12.146EPCh. 12 - Prob. 12.147EPCh. 12 - Prob. 12.148EP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The following 'H NMR spectrum was taken with a 750 MHz spectrometer: 1.0 0.5 0.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 ' 2.0 1.0 0.0 (ppm) What is the difference Av in the frequency of RF ac Δν ac radiation absorbed by the a and c protons? (Note: it's not equal to the difference in chemical shifts.) Round your answer to 2 significant digits, and be sure it has an appropriate unit symbol. = O O a will shift left, c will shift right. O a will shift right, c will shift left. a and c will both shift left, with more space between them. Suppose a new spectrum is taken with a 500 MHz spectrometer. What will be true about this new spectrum? O a and c will both shift left, with less space between them. O a and c will both shift right, with more space between them. O a and c will both shift right, with less space between them. Which protons have the largest energy gap between spin up and spin down states? O None of the above. ○ a Ob Explanation Check C Ar B 2025 McGraw Hill LLC. All Rights Reserved.…arrow_forwardWhat mass of Na2CO3 must you add to 125g of water to prepare 0.200 m Na2CO3? Calculate mole fraction of Na2CO3, mass percent, and molarity of the resulting solution. MM (g/mol): Na2CO3 105.99; water 18.02. Final solution density is 1.04 g/mL.arrow_forward(ME EX2) Prblms Can you please explain problems to me in detail, step by step? Thank you so much! If needed color code them for me.arrow_forward
- Experiment #8 Electrical conductivity & Electrolytes. Conductivity of solutions FLINN Scientific Scale RED LED Green LED LED Conductivity 0 OFF OFF 1 Dim OFF 2 medium OFF 3 Bright Dim Low or Nowe Low Medium High 4 Very Bright Medium nd very high AA Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ SE=Strong Electrolyte, FE = Fair Electrolyte CWE = Weak Electrolyte, NE= Noni Electrolyte, #Solutions 1 0.1 M NaCl 2/1x 102 M NaCl, 3/1X103 M Nall Can Prediction M Observed Conductivity Very bright red Bright red Dim red you help me understand how I'm supposed to find the predictions of the following solutions? I know this is an Ionic compound and that the more ions in a solution means it is able to carry a charge, right? AAAA Darrow_forward(SE EX 2) Prblsm 4-7: Can you please explain problems 4-7 and color code if needed for me. (step by step) detail explanationsarrow_forward(SE EX 2) Problems 8-11, can you please explain them to me in detail and color-code anything if necessary?arrow_forward
- (ME EX2) Problems 15-16 Could you please explain problems 15 through 16 to me in detail, step by step? Thank you so much! If necessary, please color-code them for me.arrow_forward1.)show any electrophilic aromatic substitution, identify the electriphile, nucleophile and transition statearrow_forward(SE EX 2) Problems 15-16, can you please explain them to me in detail and color-code anything if necessary?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License