
Concept explainers
Interpretation:
The bond line in the given structural formula represents what has to be identified from the given set of options.
Concept Introduction:
The organic compounds can be represented in two-dimensional structural format known as Structural Formula. From this we can identify the bonding between various atoms present in the molecule. Condensed structural formula and expanded structural formula are two types of structural formulas.
Expanded structural formula is the one in which all the atoms in molecule and all bonds connecting the atoms are shown clearly. If a molecule is represented using expanded structural formula means more space will be occupied.
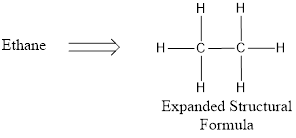
Condensed structural formula basically uses grouping of atoms. The central atom and the atoms that are connected to them are considered as groups and they are written. This is a very short hand representation of structural formula.
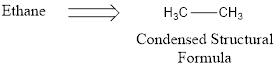
The bond line present in the above representation denotes a carbon‑carbon bond present in it.
Skeletal structural formula is also used in
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
General, Organic, and Biological Chemistry
- Draw the structure of the product of the reaction given the IR and MS data. Spectral analysis of the product reveals: MS: M 150, M-15, M-43 CH.COCI AICI, IR: 3150-3000 cm, 2950-2850 cm and 1700 cmarrow_forwardPart II. Identify whether the two protons in blue are homotopic, enantiopic, diasteriotopic, or heterotopic. a) HO b) Bri H HH c) d) H H H Br 0arrow_forwardNonearrow_forward
- Choose the option that is decreasing from biggest to smallest. Group of answer choices: 100 m, 10000 mm, 100 cm, 100000 um, 10000000 nm 10000000 nm, 100000 um, 100 cm, 10000 mm, 100 m 10000000 nm, 100000 um, 10000 mm, 100 cm, 100 m 100 m, 100 cm, 10000 mm, 100000 um, 10000000 nmarrow_forwardQ1. (a) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH3. Use curved arrows to show the electron movement. (b) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH4*. Use curved arrows to show the electron movement.arrow_forwardWhich is NOT the typical size of a bacteria? 1000 nm 0.001 mm 0.01 mm 1 umarrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,

