
(a)
Interpretation:
To predict the products of the following transesterification reaction.
Concept Introduction:
Transesterification is the process of formation of a new ester molecule from the reaction of alcohol and an ester. This is like hydrolysis of ester but here nucleophile is alcohol molecule instead of
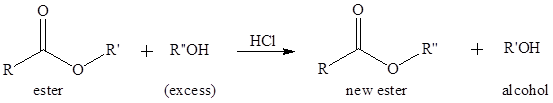
(b)
Interpretation:
To predict the products of the following transesterification reaction.
Concept Introduction:
Transesterification is the process of formation of a new ester molecule from the reaction of alcohol and an ester. This is like hydrolysis of ester but here nucleophile is alcohol molecule instead of
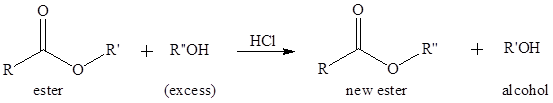
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Essential Organic Chemistry, Global Edition
- What reagents would you use to convert methyl propanoate to the following compounds? a. isopropyl propanoate b. sodium propanoate c. N-ethylpropanamide d. propanoic acidarrow_forwardIdentify the best reagents to complete the following reaction. Options are included.arrow_forwardWhich of the following reactions will result in the formation of an acyl halide? Select one: a. The reaction of a carboxylic acid with phosphorus trichloride. b. The treatment of an alcohol with ethyl bromide. c. The reaction of an ester with hydrochloric acid. d. The addition of an alkene to dilute hydrochloric acid.arrow_forward
- What is the major organic product obtained from the following reaction? A. aldehyde B. alcohol C. anhydride D. carboxylic acidarrow_forwardChoose the best reagents to complete the following reaction.arrow_forwardWhat are the composition of the following reagents? a. Tollen's reagent b. Fehling's A c. Fehling's B d. Schiff's reagent e. KMnO4arrow_forward
- From which starting materials can the product formed as a result of the following reaction be synthesized? A. Acetophenone and butanal B. Benzaldehyde and 3-pentanone C. Benzaldehyde and 2-pentanone D. Acetophenone and 2-butanonearrow_forwardWhat reactions and reagents can be used to make phenol from benzene if electrophilic aromatic substitution reactions are excluded and benzene is the only source of carbon?arrow_forwardPhosgene (COCl2) was used as a poison gas in World War I. What product would be formed from the reaction of phosgene with each of the followingreagents?a. one equivalent of methanol b. excess methanol c. excess propylamine d. excess waterarrow_forward
- Consider the structure of pent-2-ene, if it undergoes ozonolysis, which of the following final product is formed? a.Ethanal and Propanal b.Ethanal and Propanone c.CO2 and Propanal d.Ethanal and CO2arrow_forwardGive the product for each step in the reactionarrow_forwardBy means of a suitable reaction, show how each of the compounds can be prepared from propionic acid. More than one step may be required. a. methyl propylamine (CH3NHCH2CH2CH3)b. propionyl chloridec. ethyl propionated. propionic anhydridee. N-methyl propionamidearrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

