
(a)
Interpretation:
The acyl chloride and carboxylate ion that could be used to form the given mixed anhydride have to be found.
Concept Introduction:
Mixed anhydrides are unsymmetrical anhydrides which have different alkyl groups.
Example:
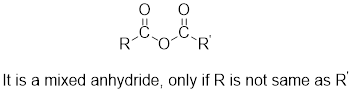
The acetyl chloride and carboxylate ion that could be used to form any given mixed anhydride can be analysed using the simple retro analysis as shown here:
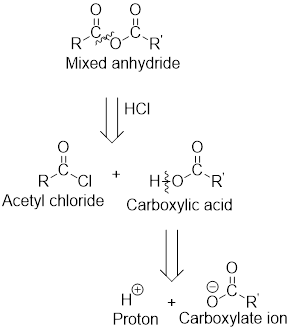
(b)
Interpretation:
The other reagents that could be used to form the given mixed anhydride have to be found.
Concept Introduction:
Mixed anhydrides are unsymmetrical anhydrides which have different alkyl groups.
Example:
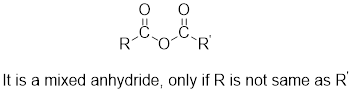
Any anhydride can be obtained using the following two pairs of reagents:
- 1. Acetyl chloride and a carboxylate ion.
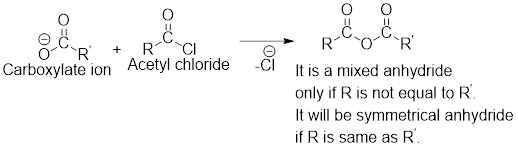
- 2. Two different carboxylic acids if mixed or unsymmetrical anhydride has to be formed or two same carboxylic acids if symmetrical anhydride has to be formed.
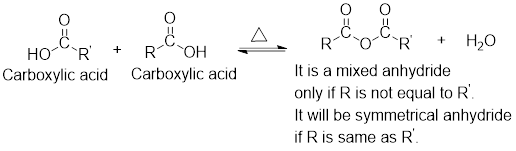
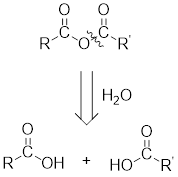
For any simple anhydride, the two carboxylic acids from which it has been formed can be found by the simple retro analysis as shown here:
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Essential Organic Chemistry, Global Edition
- Given that your mixture containing benzoic acid and aminobenzene dissolved in n-hexane solvent. Suggest a method on how to separate these two substances. Available reagents: A. Hydrochloric acid B. Sodium hydroxide C. Distilled water D. n-hexanearrow_forwardA certain compound is known to contain an aromatic benzene ring but failed to produce a fragrant yellow solution upon subjecting it to the nitration test. What may be a possible explanation for this? A. The benzene ring is part of a highly conjugated, blue dye molecule. B. The benzene ring contains a strong electron-withdrawing group. C. The benzene ring has no available sites left for electrophilic attack. D. All of the given. Kindly explain your answer in detail.arrow_forwardExplain how much excess acid was used to make the "nitrous acid" HONO that will make the diazo salt with the primary amine?arrow_forward
- Answer the questions about Resorcinol. 1. Draw the structure of organic compound assigned to you using Chemsketch. 2. Name and Draw the structures of all possible chemical (Electrophilic aromatic substitution) reactions of the compound namely; a. Halogenation (Chlorination or Bromination) b. Nitration c. Sulphomation d. Friedal Craft Alkylation e. Friedal Craft Acylationarrow_forwardFor the structure shown at the right, what type of compound is it? A. an ylide B. a nitrile C. a cyanohydrin D. an imine E. None of the selections are correctarrow_forward1. Give the product of the reaction of the cyclic anhydride shown below when treated with the following reagents. a. aquous NaOH b. aqueous HCI c. methanol d. sodium methoxide in methanol e. ethylaminearrow_forward
- 1) Answer the following questions: a. Explain oxidative and reductive Ozonolysis. b. Give the sequence of the relative reactivities of carboxylic acid derivatives. Explain why acyl chloride can be converted into ester but ester cannot be converted into acyl chloride? c. Write the product when ester of propionic acid, propionyl chloride and propanal reacts with excess of methyl magnesium bromidearrow_forward46. Which carbonyl group of the given compound is most reactive for nucleophilic addition reaction? A. Carbonyl Group 1 B. Carbonyl Group 2 C.Carbonyl Group 3 D. All have equal reactivity 47. Which of the following does NOT give a brick red precipitate with Fehling solution? A Acetone B. Acetaldehyde C. Formalin D. D-glucose 48. Which of the following structures contains a hemiacetal group CO OH H₂C 0 OCH, A OH OCH H₂C-C-CH₂ C C.H. CH OCH OCH, barrow_forwardExplain why acetyl chloride reacts faster with water than acetic anhydride does?arrow_forward
- Which of these statements is NOT true about the reaction of amines with a cyclic anhydride like phthalic anhydride? a. Dimethylamine will give a cyclic imide product. b. Aniline will give a cyclic imide product. c. Triethylamine will give a cyclic imide product. d. The reaction undergoes a nucleophilic acyl substitution mechanism.arrow_forward1. Put these three common types of carbonyl compound in order of decreasing reactivity ester amide acid chloride 2. For the least reactive, show the interconversion to its other resonance form: How does this electron delocalisation make it stable? 3. For the most reactive, draw the mechanism of its undergoing hydrolysis (reaction with H2O): Why makes this type of carbonyl so reactive to nucleophiles?arrow_forward2. Name and Draw the structures of all possible chemical (Electrophilic aromatic substitution) reactions of the Aniline; a. Halogenation (Chlorination or Bromination) b. Nitration c. Sulphonation d. Friedal Craft Alkylation e. Friedal Craft Acylationarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





