
Concept explainers
(a)
Interpretation:
The given skeletal structure needs to be converted into complete structure with all the carbon and hydrogen and lone pairs on the hetero-atoms.
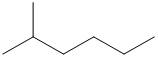
Concept Introduction:
Skeletal formula is also called as shorthand formula. In
Complete structural formula of a molecule represents all the atoms of molecule, types of bonds connecting atoms and how atoms are connected to each other.
(b)
Interpretation:
The given skeletal structure needs to be converted into complete structure with all the carbon and hydrogen and lone pairs on the hetero-atoms.
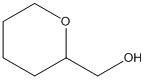
Concept Introduction:
Skeletal formula is also called as shorthand formula. In organic chemistry is a form of representation in which only molecular bonding and molecular geometry is shown. Carbon and hydrogen atom is common in organic chemistry. Carbon atoms are generally shown by vertices of the bonds. Unlabelled vertex will represent carbon atom attached to number of hydrogen atom s required to fulfill its valency (i.e. four).
Complete structural formula of a molecule represents all the atoms of molecule, types of bonds connecting atoms and how atoms are connected to each other.
(c)
Interpretation:
The given skeletal structure needs to be converted into complete structure with all the carbon and hydrogen and lone pairs on the hetero-atoms.
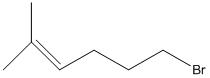
Concept Introduction:
Skeletal formula is also called as shorthand formula. In organic chemistry is a form of representation in which only molecular bonding and molecular geometry is shown. Carbon and hydrogen atom is common in organic chemistry. Carbon atoms are generally shown by vertices of the bonds. Unlabelled vertex will represent carbon atom attached to number of hydrogen atom s required to fulfill its valency (i.e. four).
Complete structural formula of a molecule represents all the atoms of molecule, types of bonds connecting atoms and how atoms are connected to each other.
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
General, Organic, and Biological Chemistry - 4th edition
- bre The reaction sequence shown in Scheme 5 demonstrates the synthesis of a substituted benzene derivative Q. wolsd works 2 NH2 NaNO2, HCI (apexe) 13× (1 HNO3, H2SO4 C6H5CIN2 0°C HOTE CHINO₂ N O *O₂H ( PO Q Я Scheme 5 2 bag abouoqmics to sounde odi WEIC (i) Draw the structure of intermediate O. [2 marks] to noitsmot od: tot meinedogm, noit so oft listsb ni zaupaib bas wa (ii) Draw the mechanism for the transformation of aniline N to intermediate O. Spoilage (b) [6 marks] (iii) Identify the reagent X used to convert compound O to the iodinated compound [tom E P. vueimado oilovonsa ni moitos nolisbnolov ayd toes ai tedw nisiqx (iv) Identify the possible structures of compound Q. [2 marks] [2 marks] [shom 2] (v) bus noires goiribbeolovo xnivollot adj to subora sidab Draw the mechanism for the transformation of intermediate P to compound Q. [5 marks] vi (vi) Account for the regiochemical outcome observed in the reaction forming compound Q. [3 marks]arrow_forwardPROBLEM 4 Solved Show how 1-butanol can be converted into the following compounds: a. PROBLEM 5+ b. d. -C= Narrow_forwardWhich alkene is the major product of this dehydration? OH H2SO4 heatarrow_forward
- Please correct answer and don't used hand raiting and don't used Ai solutionarrow_forwardPlease correct answer and don't used hand raitingarrow_forwardThe vibrational contribution isa) temperature independent for internal energy and heat capacityb) temperature dependent for internal energy and heat capacityc) temperature independent for heat capacityd) temperature independent for internal energyarrow_forward
- Quantum mechanics. Explain the basis of approximating the summation to an integral in translational motion.arrow_forwardQuantum mechanics. In translational motion, the summation is replaced by an integral when evaluating the partition function. This is correct becausea) the spacing of the translational energy levels is very small compared to the product kTb) the spacing of the translational energy levels is comparable to the product kTc) the spacing of the translational energy levels is very large compared to the product kTarrow_forwardDon't used Ai solutionarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





