
Concept explainers
(a)
Interpretation:
To recognize the functional group / groups in the following compound:
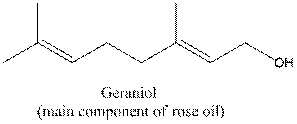
Concept Introduction:
Organic molecules have some structural features in addition to
Table given below gives information about some common functional groups:
| Type of Compound | General Structure |
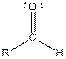 | |
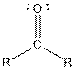 | |
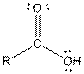 | |
| Ester | 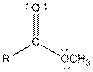 |
| Amide | 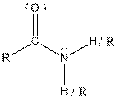 |
| Alcohol |  |
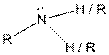 | |
 |
Here, R = any carbon backbone
Complete structural formula of a molecule represents all the atoms of molecule, types of bonds connecting atoms and how atoms are connected to each other.
(b)
Interpretation:
To recognize the functional group / groups in the following compound:
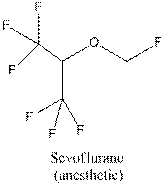
Concept Introduction:
Organic molecules have some structural features in addition to
Table given below gives information about some common functional groups:
| Type of Compound | General Structure |
| Aldehyde | 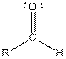 |
| Ketone | 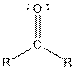 |
| Carboxylic acid | 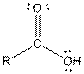 |
| Ester | 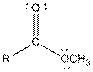 |
| Amide | 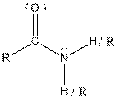 |
| Alcohol |  |
| Amine | 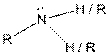 |
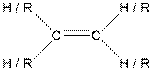
| Alkene | 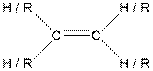 |
| Ether | 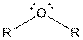 |
Here, R = any carbon backbone
Complete structural formula of a molecule represents all the atoms of molecule, types of bonds connecting atoms and how atoms are connected to each other.
(c)
Interpretation:
To recognize the functional group / groups in the following compound:
Concept Introduction:
Organic molecules have some structural features in addition to
Table given below gives information about some common functional groups:
| Type of Compound | General Structure |
| Aldehyde | 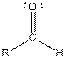 |
| Ketone | 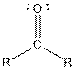 |
| Carboxylic acid | 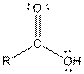 |
| Ester | 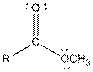 |
| Amide | 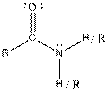 |
| Alcohol | 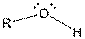 |
| Amine | 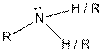 |
| Alkene | 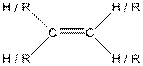 |
Here, R = any carbon backbone
Complete structural formula of a molecule represents all the atoms of molecule, types of bonds connecting atoms and how atoms are connected to each other.
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
General, Organic, and Biological Chemistry - 4th edition
- What are the functional groups present in each compound?arrow_forwardWrite the letter of the pair of compounds that illustrates the given isomerism. CHS CH H3C CH, OH A B HC но H,C. CHs H,C. H,C. Br CHS он CIE F CH3 Br H3C. H3C. CH3 CH CH3 NH2 CH L AND Functional Isomerism:arrow_forward= NAMING AND DRAWING ORGANIC MOLECULES Drawing a skeletal structure from a condensed structure Draw a skeletal ("line") structure of this molecule: CH3 OH -6-6- CH3-CH₂-C=C-CH3 Click and drag to start drawing a structure.arrow_forward
- identify the functional group of each compound.arrow_forwardWhat is the difference between a structural isomer and structural conformations of an organic molecule?arrow_forwardCH2-CH3 у. Type of functional group CH2-CH3 Common Name 1 CH2-CH3 Common Name 2 (Derived Name) z. Type of functional group IUPAC Derived Namearrow_forward
- Draw skeletal structures for the compounds including any cis–trans isomers.arrow_forwardDraw a skeletal ("line") structure of this molecule: CH3 CH3 C-CH2–0-CH3arrow_forwardBased on the structures below: i) Convert the structures above to the skeletal structure.ii) Identify the functional groups and homologous series in each compound.arrow_forward
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning



