
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please correct answer and don't used hand raiting
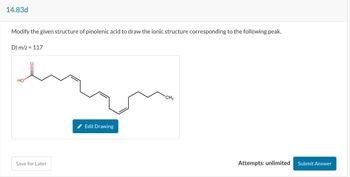
Transcribed Image Text:14.83d
Modify the given structure of pinolenic acid to draw the ionic structure corresponding to the following peak.
D) m/z = 117
HO
Save for Later
Edit Drawing
CH3
Attempts: unlimited Submit Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- [Review Topics] Determine a molecular formula, e.g. CH4, from the line structure below. Specify elements in the following order: C, H, others(in alphabetical order). Example: C4H7CIOS Molecular formula C. C₁ 5 Submit Answer Retry Entire Group 4 more group attempts remaining [Re Ć [Review Topics] Determine a molecular formula, e.g. CH4, from the line structure below. Specify elements in the following order: C, H, others(in alphabetical order). Example: C4 H7CIOS Molecular formula C [Rearrow_forwardPlease don't provide handwriting solutionarrow_forwardWhich corresponds to the best answer?arrow_forward
- Page 19 of 19 6. What molecular ion(s) would you expect for compounds having the molecular formulas Example) CH-F A. C.H.CI B. CHIN C. CH4N₂ D. E. F. Br i C₁H₂F = 62 One peak at m/z 62arrow_forward3.What peaks would expect to find for you Vs, cie'thy) amine thy) in the InfraRed' spectrum? +-butyl 'amine us. n-butyl aminearrow_forwardRelative Abundance IR Peaks A B NMR DUR SA 1997 STRUCTURE TROBLEM VA D (tuo) E (two) MASS SPECTRUM: -- с INTEGRAL-S MASS SPECTRUM m/e 91 43 29 B INTEGRAL Ca 10 PF www STRUCTURE Your Same Assignmeuty NAR A M(134) 7pm 5 Spetutting no. of PROPOSED STRUCTURE: Please propose a structure that fits SODAR, IR, NMR, and Mass Spectrum. Also, Cirse each group of hydrogens elabel them A, B, C to show the NMR assignments. 43 99arrow_forward
- Which atom do you expect is present in this spectrum? 100 Rel. Intensity 80 60 60 10 40 20 20 0.0 0.0 15 30 45 60 60 m/z NIST Chemistry WebBook (https://webbook.nist.gov/chemistry) O Only hydrocarbons O Bromine O Chlorine ○ Nitrogen 75 90arrow_forwardFinal answer in skeletal formarrow_forwardConsider the following molecule, draw the structure of the fragment that you would expect to be the base peak in the MS. .. NN []n ? HOCX D Marvin JS by ChemAxon H C N O SF P CI Br Open in Rearrow_forward
- Question in photoarrow_forwardPlease explain how to solve the following!arrow_forwardThe nitrogen rule of mass spectrometry says that a compound containing an odd number of nitrogens has an odd-numbered molecular ion. Conversely, a compound containing an even number of nitrogens has an even-numbered M+ peak. Explain.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
