
Concept explainers
(a)
Interpretation: The type of coal that contains large percent mass of carbon needs to be determined using the given charts.
Concept Introduction : The substance produced from dead plants and animal remains which are buried and go through high pressure and heat over a long time period is coal. Coal is a solid fossil fuel consisting of carbon. There are various types of coal, such as, lignite, bituminous etc.
(a)
Answer to Problem 2RQ
Bituminous coal has large percent mass of carbon.
Explanation of Solution
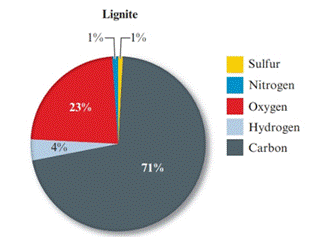
Given pie chart for the Lignite coal is as follows:
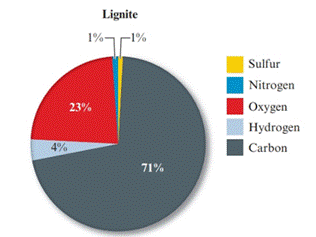
Given pie chart for the Bituminous coal is as follows:
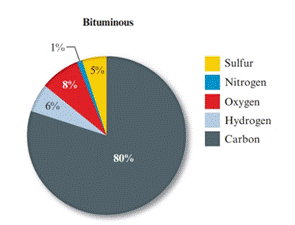
In the lignite coal, percent of carbon is 71% and in the bituminous coal, percent of carbon is 80%. So, the coal having large percent mass of carbon is bituminous coal.
(b)
Interpretation: The type of coal that contains large percent mass of sulphur needs to be determined using the given charts.
Concept Introduction : The substance produced from dead plants and animal remains which are buried and go through high pressure and heat over a long time period is coal. Coal is a solid fossil fuel consisting of carbon. There are various types of coal, such as, lignite, bituminous etc.
(b)
Answer to Problem 2RQ
Coal that contains large percent mass of sulphur is bituminous coal.
Explanation of Solution
Given pie chart for the Lignite coal is as follows:
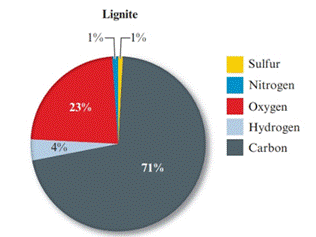
Given pie chart for the Bituminous coal is as follows:
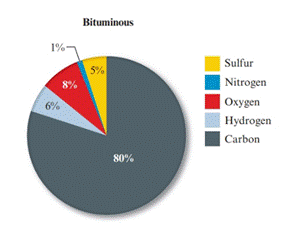
In the lignite coal, percent of sulphur is 1% and in the bituminous coal, percent of carbon is 5%. So, the coal having large percent mass of sulphur is bituminous coal.
(c)
Interpretation: The element present in equal amounts in both the types of carbon (coal) needs to be determined.
Concept Introduction : The substance produced from dead plants and animal remains which are buried and go through high pressure and heat over a long time period is coal. Coal is a solid fossil fuel consisting of carbon. There are various types of coal, such as, lignite, bituminous etc.
(c)
Answer to Problem 2RQ
Nitrogen is present in equal amounts in both the coal.
Explanation of Solution
Given pie chart for the Lignite coal is as follows:
Given pie chart for the Bituminous coal is as follows:
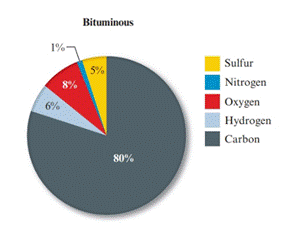
The percentage of nitrogen in both the coal (lignite and bituminous) is 1%. So, nitrogen is present in equal amount in both the coal.
Chapter 10 Solutions
World of Chemistry, 3rd edition
- 10. (5pts) Provide the complete arrow pushing mechanism for the chemical transformation → depicted below Use proper curved arrow notation that explicitly illustrates all bonds being broken, and all bonds formed in the transformation. Also, be sure to include all lone pairs and formal charges on all atoms involved in the flow of electrons. CH3O II HA H CH3O-H H ①arrow_forwardDo the Lone Pairs get added bc its valence e's are a total of 6 for oxygen and that completes it or due to other reasons. How do we know the particular indication of such.arrow_forwardNGLISH b) Identify the bonds present in the molecule drawn (s) above. (break) State the function of the following equipments found in laboratory. Omka) a) Gas mask b) Fire extinguisher c) Safety glasses 4. 60cm³ of oxygen gas diffused through a porous hole in 50 seconds. How long w 80cm³ of sulphur(IV) oxide to diffuse through the same hole under the same conditions (S-32.0.0-16.0) (3 m 5. In an experiment, a piece of magnesium ribbon was cleaned with steel w clean magnesium ribbon was placed in a crucible and completely burnt in oxy cooling the product weighed 4.0g a) Explain why it is necessary to clean magnesium ribbon. Masterclass Holiday assignmen PB 2arrow_forward
- Hi!! Please provide a solution that is handwritten. Ensure all figures, reaction mechanisms (with arrows and lone pairs please!!), and structures are clearly drawn to illustrate the synthesis of the product as per the standards of a third year organic chemistry course. ****the solution must include all steps, mechanisms, and intermediate structures as required. Please hand-draw the mechanisms and structures to support your explanation. Don’t give me AI-generated diagrams or text-based explanations, no wordy explanations on how to draw the structures I need help with the exact mechanism hand drawn by you!!! I am reposting this—ensure all parts of the question are straightforward and clear or please let another expert handle it thanks!!arrow_forwardIn three dimensions, explain the concept of the velocity distribution function of particles within the kinetic theory of gases.arrow_forwardIn the kinetic theory of gases, explain the concept of the velocity distribution function of particles in space.arrow_forward
- In the kinetic theory of gases, explain the concept of the velocity distribution function of particles.arrow_forwardHi!! Please provide a solution that is handwritten. this is an inorganic chemistry question please answer accordindly!! its just one question with parts JUST ONE QUESTION with its parts spread out till part (g), please answer EACH part till the end and dont just provide wordy explanations wherever asked for structures, please DRAW DRAW them on a paper and post clearly!! answer the full question with all calculations step by step EACH PART CLEARLY please thanks!! im reposting this please solve all parts and drawit not just word explanations!!arrow_forwardHi!! Please provide a solution that is handwritten. this is an inorganic chemistry question please answer accordindly!! its just one question with parts JUST ONE QUESTION, please answer EACH part PART A AND PART B!!!!! till the end and dont just provide wordy explanations wherever asked for structures, please DRAW DRAW them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this please solve all parts and drawit not just word explanations!!arrow_forward
- Hi!! Please provide a solution that is handwritten. this is an inorganic chemistry question please answer accordindly!! its just one question with parts JUST ONE QUESTION, please answer EACH part till the end and dont just provide wordy explanations wherever asked for structures, please DRAW DRAW them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this please solve all parts and drawit not just word explanations!!arrow_forward8b. Explain, using key intermediates, why the above two products are formed instead of the 1,2-and 1,4- products shown in the reaction below. CIarrow_forward(5pts) Provide the complete arrow pushing mechanism for the chemical transformation depicted below Use proper curved arrow notation that explicitly illustrates all bonds being broken, and all bonds formed in the transformation. Also, be sure to include all lone pairs and formal charges on all atoms involved in the flow of electrons. CH3O H I I CH3O-H H I ① Harrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





