
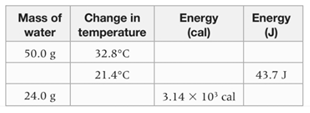
Interpretation: The given table needs to be completed.
Concept introduction: The formula to calculate energy is,
Where Q is energy, s is specific heat capacity and
Answer to Problem 2RQ
The complete table is as follows:
| Mass of water | Change in temperature | Energy (cal) | Energy (J) |
| 50.0 g | 6861.76 J | 1640 cal | |
| 0.488 g | 10.44 cal | 43.7 J | |
| 24.0 g |
Explanation of Solution
For case 1:
Mass of water is 50 g and temperature difference is
Put all the values in equation (1).
So, energy in joule is 6861.76 J
So, conversion factor to convert energy from joule to calorie is is
So, energy in calorie is 1640 cal.
For case 2:
Change in temperature is
So, mass of water is 0.488 g.
So, conversion factor to convert energy from joule to calorie is is
So, energy in calorie is 10.44 cal.
For case 3:
Mass of water is 24.0 g and energy in calorie is
Conversion factor to convert energy from calorie to joule is
So,
So, energy in Joule is
Now use equation (1) to calculate change in temperature.
So, temperature difference is
Joule and Calorie both are units of energy.
Chapter 10 Solutions
World of Chemistry, 3rd edition
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





