
(a)
Interpretation: The entropy change for the given process needs to be explained.
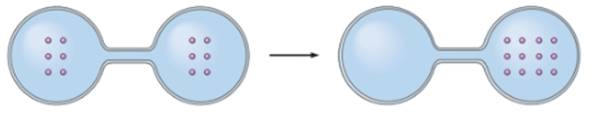
Concept Introduction: The particles of a system remain in motion continuously. The randomness in a system is denoted by entropy of system. As the disorder increases in a system, the entropy of system also increases.
(a)
Answer to Problem 45A
The entropy of given process decreases.
Explanation of Solution
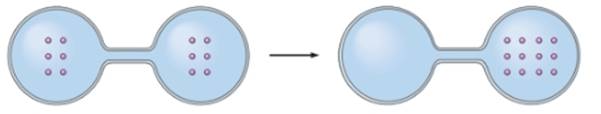
In the given image, the particles diffuse in same flask. It decreases the movement of particles as number of particles increases in one flask. Thus, it decreases the entropy of system.
(b)
Interpretation: The entropy change for the given process needs to be explained.
Concept Introduction: The particles of a system remain in motion continuously. The randomness in a system is denoted by entropy of system. As the disorder increases in a system, the entropy of system also increases.
(b)
Answer to Problem 45A
The entropy of given process increases.
Explanation of Solution
In the given reaction, solid
Thus, it increases the randomness of system, which further increases the entropy of system.
(c)
Interpretation: The entropy change for the given process needs to be explained.
Concept Introduction: The particles of a system remain in motion continuously. The randomness in a system is denoted by entropy of system. As the disorder increases in a system, the entropy of system also increases.
(c)
Answer to Problem 45A
The entropy of given process decreases.
Explanation of Solution
In the given reaction, two gases combine to form liquid water. The entropy of liquid is lesser than gas as they have strong intermolecular force of attraction between particles.
Thus, it decreases the randomness of system, which further decreases the entropy of system.
(d)
Interpretation: The entropy change for the given process needs to be explained.
Concept Introduction: The particles of a system remain in motion continuously. The randomness in a system is denoted by entropy of system. As the disorder increases in a system, the entropy of system also increases.
(d)
Answer to Problem 45A
The entropy of given process increases.
Explanation of Solution
In the given reaction, evaporation of liquid water to gaseous water. The entropy of liquid is lesser than gas as they have strong intermolecular force of attraction between particles.
Thus, it increases the randomness of system, which further increases the entropy of system.
Chapter 10 Solutions
World of Chemistry, 3rd edition
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





