
Concept explainers
(a)
Interpretation :
The given samples must be listed in order of increasing gas pressure.
Concept Introduction :
A gas sample with lesser volume and larger number of molecules will have high pressure as per ideal gas equation. The equation is as follows:
Here P, V,n, R and T are pressure, volume, mole number, universal gas constant and absolute temperature respectively.
(a)
Answer to Problem 6E
Sample in the order of increasing gas pressure will be
sample B <sample A<sample C.
Explanation of Solution
Sample C has maximum number of gas molecules and smallest volume. Thus, pressure will be maximum.
Sample B and C have same number of gas molecules but sample B has larger volume as compared to sample A. Thus, sample B will have minimum pressure.
b)
Interpretation :
A volume of air must be sketched that has a pressure in between the pressures in boxes A and B.
Concept Introduction :
To have in between pressure of a sample with respect to box A and B, the
b)
Answer to Problem 6E
A sketch of air is shown below.
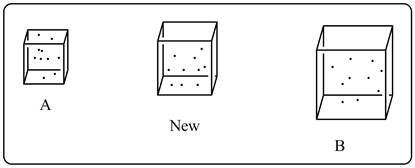
Explanation of Solution
From the sketch it is clear that the new sample also contains same number of gas molecules (10) as present in box A and B. But the volume of new sample is more than sample A but less then sample B. Thus
Chapter U3 Solutions
Living by Chemistry
Additional Science Textbook Solutions
Campbell Essential Biology with Physiology (5th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
College Physics: A Strategic Approach (3rd Edition)
Chemistry: The Central Science (14th Edition)
Human Biology: Concepts and Current Issues (8th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
- Please correct answer and don't used hand raitingarrow_forwardThe vibrational contribution isa) temperature independent for internal energy and heat capacityb) temperature dependent for internal energy and heat capacityc) temperature independent for heat capacityd) temperature independent for internal energyarrow_forwardQuantum mechanics. Explain the basis of approximating the summation to an integral in translational motion.arrow_forward
- Quantum mechanics. In translational motion, the summation is replaced by an integral when evaluating the partition function. This is correct becausea) the spacing of the translational energy levels is very small compared to the product kTb) the spacing of the translational energy levels is comparable to the product kTc) the spacing of the translational energy levels is very large compared to the product kTarrow_forwardDon't used Ai solutionarrow_forwardPlease correct answer and don't used hand raiting don't used Ai solutionarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





