
Concept explainers
(a)
Interpretation: Reason behind
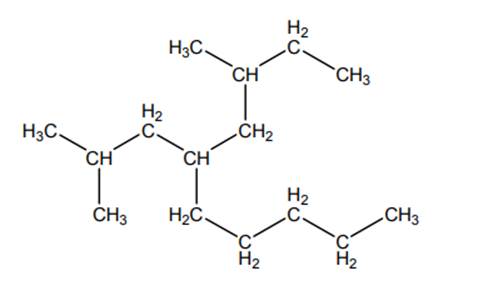
Concept introduction:The IUPAC name begins with prefix to designate the number of
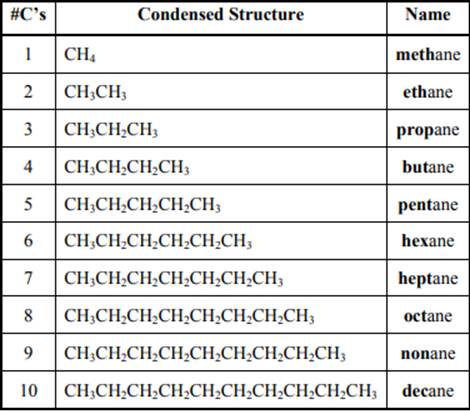
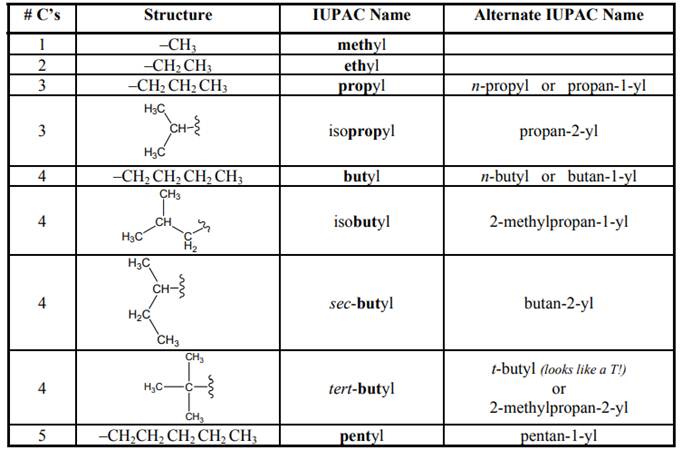
The IUPAC system for nomenclature of straight hydrocarbon makes use of table given as follows:
As per IUPAC recommendations, longest chain found in a continuous manner in a branched molecule is chosen as parent chain.
(b)
Interpretation:Reason behind
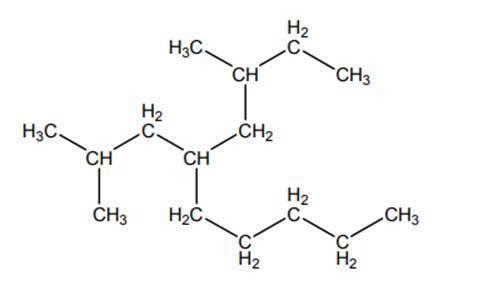
Concept introduction:The IUPAC name begins with prefix to designate the number of
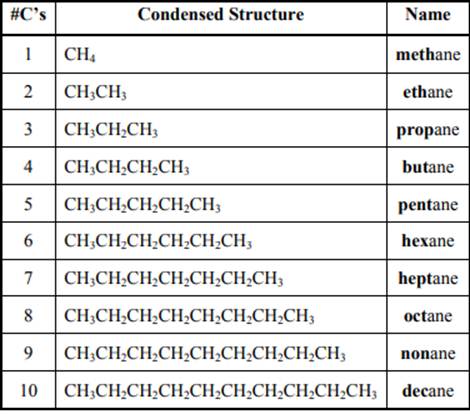
The IUPAC system for nomenclature of straight hydrocarbon makes use of table given as follows:
As per IUPAC recommendations, all the side chains are named in alphabetical order.Names of branches that occur commonly as side chains are listed below:
Trending nowThis is a popular solution!

Chapter NW1 Solutions
Organic Chemistry: A Guided Inquiry
- (CQ2) Hello! Please help me answer this one. Please refer to the given picture/s below for the questions. Please read the instructions and directions very carefully. Double and triple check your answers, previous tutors got it wrong. NOTE: Type only your answers. Please do not handwritten your answers. Make sure your formulas, solutions and answers' format are all correct. ANSWER ALL THE NUMBERS 1-5 ONLY!arrow_forwardChemistry Questionarrow_forward(CQ4) Hello! Please help me answer this one. Please refer to the given picture/s below for the questions. Please read the instructions and directions very carefully. Double and triple check your answers, previous tutors got it wrong. NOTE: Type only your answers. Please do not handwritten your answers. Make sure your formulas, solutions and answers' format are all correct.arrow_forward
- First, add curved arrow(s) to show the resonance using the following pattern: an allylic carbocation. Modify the second structure given to draw the new resonance structure. Include lone pairs and charges in your structure. Use the + and - tools to add charges to an atom, and use the single bond tool to add/remove double bonds. .CH3 .CH3 ČH3arrow_forward1. Draw ethylbenzene and put a + on the carbon next to the benzene (innermost carbon in ethyl group). Show all resonance forms for this ion. 2. draw the naphthalene and put one - on the neighboring carbon of the "crossroads". show every resonance forms for this ion. 3. Draw benzoic acid and draw on all p-orbitals.arrow_forward(CQ8) Hello! Please help me answer this one. Please refer to the given picture/s below for the questions. Please read the instructions and directions very carefully. Double and triple check your answers, previous tutors got it wrong. NOTE: Type only your answers. Please do not handwritten your answers. Make sure your formulas, solutions and answers' format are all correct. Answer all the questions, please copy the format in the given picture in answering this!arrow_forward
- (E) What suffix do all the names in Model 1 have in common with each other?arrow_forwardUse Model 1 to propose names for three-, four-, five-, and six-carbon branches that follow the same pattern as “methyl” and “ethyl” for one- and two-carbon branches, respectively. (Note: The names of seven-, eight-, etc. carbon branches follow the same pattern, but branches of such length are rare since they are usually the parent chain.)arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
