
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter NW1, Problem 12CTQ
Interpretation Introduction
Interpretation: The information conveyed by “di” and “tri” in below figure should be given.
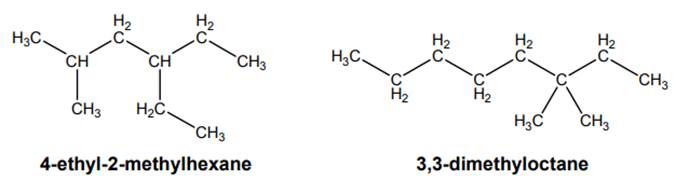
Concept introduction: The IUPAC name begins with prefix to designate the number of
The IUPAC system for nomenclature of straight hydrocarbon makes use of table given as follows:
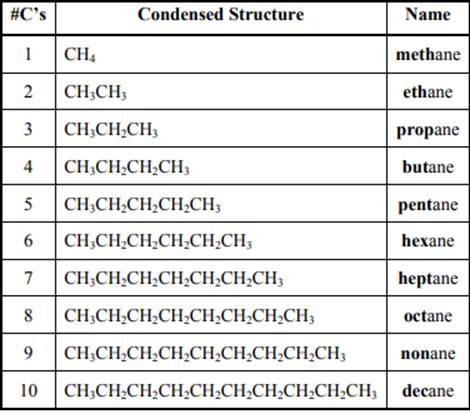
As per IUPAC recommendations, longest chain found in a continuous manner in a branched molecule is chosen as parent chain. All the side chains are named in alphabetical order.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Directions: Given the compounds on the 1st column, check which test/s would it give positive results. The first compound is done for you as an example.
I need help with these questions
(Not honor class)
(Not grading)
How does pentane’s boiling point compare with the boiling points of the alcohol and the aldehyde in Model 1?
Can molecular weight differences account for the observed differences in boiling points?
Propose an explanation for the observed differences in boiling points.
Compare the boiling points of the ketone and carboxylic acid. Propose an explanation for the observed differences in boiling points.
Why do carboxylic acids have the highest boiling point, even higher than alcohols? explain this observation.
Chapter NW1 Solutions
Organic Chemistry: A Guided Inquiry
Ch. NW1 - Prob. 1CTQCh. NW1 - (E) Write a correct name below each of the...Ch. NW1 - (E) What suffix do all the names in Model 1 have...Ch. NW1 - (E) What prefix stands for eight carbons?Ch. NW1 - Prob. 5CTQCh. NW1 - Prob. 6CTQCh. NW1 - Prob. 7CTQCh. NW1 - Prob. 8CTQCh. NW1 - Prob. 9CTQCh. NW1 - Use Model 1 to propose names for three-, four-,...
Ch. NW1 - Prob. 11CTQCh. NW1 - Prob. 12CTQCh. NW1 - Prob. 13CTQCh. NW1 - Name the following alkanes.Ch. NW1 - (Check your work.) Explain what is wrong with each...Ch. NW1 - Prob. 16CTQCh. NW1 - Draw the following alkanes a....Ch. NW1 - Prob. 18CTQCh. NW1 - Draw structures that correspond to the following...Ch. NW1 - For mono-substituted cycloalkanes the “1” is not...Ch. NW1 - Prob. 21CTQCh. NW1 - Prob. 22CTQCh. NW1 - Prob. 23CTQCh. NW1 - Prob. 24CTQCh. NW1 - Prob. 25CTQCh. NW1 - Write the name of the molecule on the left using...Ch. NW1 - Prob. 1ECh. NW1 - Prob. 2ECh. NW1 - Name each of the following structures.
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (CQ5) Hello! Please help me answer this one. Please refer to the given picture/s below for the questions. Please read the instructions and directions very carefully. Double and triple check your answers, previous tutors got it wrong. NOTE: Type only your answers. Please do not handwritten your answers. Make sure your formulas, solutions and answers' format are all correct. ANSWER THE LEARNING TASK 1 & 2 ONLY!arrow_forward4. If any sugar shown below is a "reducing sugar," circle its corresponding letter. H OH HO НО НО, H OH НО H Н HO с Н H HO Н ОН 0 A OH -HH OH НО H H OH HO H OH OH H НО HO Н Н НО D HO B OH H OH Н- н OH HO Н Н OH О Н НО НО Н H OH Н OHarrow_forwardPropose synthesis ! Draw out each step for these two questions instead of writting jn wordsarrow_forward
- Please step by step answer and current answer pleasearrow_forwardUse Model 1 to propose names for three-, four-, five-, and six-carbon branches that follow the same pattern as “methyl” and “ethyl” for one- and two-carbon branches, respectively. (Note: The names of seven-, eight-, etc. carbon branches follow the same pattern, but branches of such length are rare since they are usually the parent chain.)arrow_forwardAnswer this question very fast!arrow_forward
- The organic product in the drawing area below can be made from an alkene addition reaction that produces mostly Markovnikov product. Draw in the alkene and small molecule reactant on the left-hand side of the reaction. 0 Click and drag to start drawing a structure. + X xx Darrow_forwardComplete the generic mechanism for an electrophilic aromatic substitution (EAS) reaction using E as the electrophile, and show- how the sigma complex is resonance stabilized. Use curved arrows to show the mechanism and the conversion between resonance structures. Make sure to add any missing charges. Note the use of a generic base (B) in the last step. Then, label the reaction coordinate diagram for a typical EAS reaction by correctly placing the structures on the diagram. Step 1: add a curved arrow. H H H H H E slowarrow_forwardPlease label the organic and inorganic species so I can know which answer is to what part of the question.arrow_forward
- Draw the products of the reaction shown. Electron flow is indicated with curved arrows. CH3 H3C CH3 CH3 Include all valence lone pairs in your answer. • Include counter-ions, e.g., Na+, I, in your submission, but draw them in their own separate sketcher. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate multiple products using the + sign from the drop-down menu. 85 narrow_forwardMacmillan Learni Step 1: add curved arrows. Select Draw Templates More C H H C H to H Step 3: complete the structure and add curved arrows. Select Draw Templates More H H Erase Q 2 Q Erase 11 Step 2: complete the structure and add a curve arrow. Draw only one curved arrow. Select Draw Templates More / |||||| C H 0 ou Select Draw Templates More : 0 C H H Step 4: complete the structure and add curved arrows. Erase Q2 Q H H 01-01 Erasearrow_forwardIn the drawing area below, create an acetal with at least 3 methoxy groups, and a total of 5 carbon atoms.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning