
Concept explainers
(a)
Interpretation: Statistically the most likely compound to form a mono-bromide, for substrates 8-13, needs to be explained.
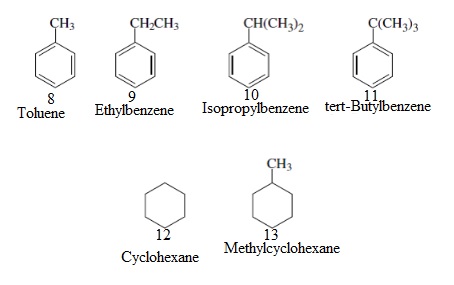
Concept Introduction: A bromide that contains only one bromine atom in the molecule is called a mono-bromide.
(b)
Interpretation: The most likely compound to form a single isomer of a monobromide for substrates 8-13, needs to be explained.
Concept Introduction: Compounds or radicals having the same type of atoms in the same numbers, and varying in properties and structures, is called a single isomer.
(c)
Interpretation: The compound that is expected to produce the most stable radical, for substrates 8-13, needs to be explained.
Concept Introduction: The neighboring elements which are rich in electrons, stabilize radicals. Thus the least stable radical will be the primary radical and the most stable radical will be tertiary radical.
(d)
Interpretation: The most compound that is expected to produce the least stable radical for substrates 8-13, needs to be explained.
Concept Introduction: Primary radicals are the least stable radicals.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
- 11? Please also address what ion of germanium is most likely to form!arrow_forwardUsing your class average data, compare the following alkyl halides for the SN1 and SN2 reactions: Br Br Why do you think these alkyl halides very reactive in SN1 and SN2 reaction?arrow_forward1. Suppose you want to study the influence of IO3- concentration on the rate of the reaction used in Part A. Which of the following procedures would be appropriate? a. Use different amounts of KIO3 solution with a constant amount of water and a constant amounts of NaHSO3 solution. b. Use different amounts of KIO3 solution, constant amounts of NaHSO3 solution, and different water amounts to give a constant total amount. c. Use different amounts of KIO3 solution, different amounts of NaHSO3 solution, and constant amounts of water. Explain your answer: The answer is B, I just need an explanation on whyarrow_forward
- a. Write two reaction paths for the production of monochlorodecane (C10H21CI) by reaction of Decane (C10H22) with molecular chlorine (Cl2). Decene (C10H20) with hydrogen chloride (HCI). b. What type of reaction occurs in both (i) and (ii) above? i. ii.arrow_forward6. The quantum yield of a photochemical reaction A to produce B was found to be 0.5. The reaction was made after irradiation of A with light of 365 nm to produce 5 mmol of B. Calculate the total energy absorbed in the reaction. 7. The electronic transition due →* of isolated double bond is appears at shorter wavelength compared with the conjugated one, explain. 8. Photosensitization is an energy transfer between two molecules, explain the difference between excitation with or without photosensitizer and give an example 9. Dye-sensitized solar cells, as well as photodynamic therapy, is an application of photosensitization explain and write the mechanism for both cases. 10. Excimer and exciplex are essential in sensing applications; write two examples with detail of their importance. 11. Photoinduced electron transfer causes fluorescence quenching in a certain chromophore, the fact that inspired researcher for designing chromophore to be used as a fluorescent sensor, explain and give an…arrow_forward4. Consider the reaction shown below. Which term best describes this reaction? a. Addition b. Elimination c. Rearrangement d. Substitution + Br₂ Br Brarrow_forward
- What is the increasing order of reactivity ?arrow_forward[References] Use the References to access important values if needed for this question. Draw a structural formula for potassium benzoate, showing the charges on the cation and the anion. O . You do not have to consider stereochemistry. . You do not have to explicitly draw H atoms. • Draw cations and anions in separate sketchers. + Submit Answer **** Ⓡ ChemDoodle Retry Entire Group ▼ Sn [F 9 more group attempts remainingarrow_forwardBond Bond energy (kcal/mol) B. Given in the table are the bond energies (in kcal/mol) of some of the bonds in the compounds involved in the free-radical chlorination of ethane. C-H 98 H-CI CI-CI 103 58 81 C-CI 1. Using these values calculate the AH of the two chain-propagating steps (steps 2 and 3). Show your calculations in the space provided below. AH step 2 ДН step 3arrow_forward
- 5. Diisobutylaluminum hydride (pictured below) reacts with nitrile A to form product B, which no longer contains a nitrile. While we have not yet seen this reaction in this course, you will be able to answer the following questions given the information below. -A. CN 1. H B 2. H30* A What change(s) in the IR spectrum are consistent with the disappearance of A as the reaction progresses? a. b. The IR spectrum and molecular formula of B are shown below. Use arrows to point out important IR stretches and label each arrow with the type of bond or functional group present. You may find some helpful information on page 7. CH120 L00 4000 3000 200 1000 HAVENUNBERI l c. Propose the structure of compound B.arrow_forwardI just was wondering how to use these equations at the top to find the H for the 3rd reaction in part b. Thanks!arrow_forwardThe standard enthalpies of formation of ClO and ClO2 are101 and 102 kJ/mol, respectively., Calculate the overallenthalpy change for each step in the following catalyticcycle: What is the enthalpy change for the overall reaction thatresults from these two steps? ClO(g) + O3(g) ---->ClO2(g) + O2(g) ClO2(g) + O(g) ---->ClO(g) + O2(g)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





