
Concept explainers
(a)
Interpretation: The reason for the bromination to be much more regioselective than chlorination needs to be explained.
Concept Introduction : Hess's law states that when the reactants are converted to products the change of enthalpy (i.e. the heat of reaction at constant pressure) does not dependent on the pathway between the initial and final states.
Hess’s law states that enthalpy changes are additive. Thus, for a single reaction,
(a)
Answer to Problem 10E
The radical construction depends on the statistical factor and bromination reaction the reaction is endothermic and hence the transition state is nearer to the radical generated than the
The formation of radical depends on the stability of the radical and more selectivity is attained .
Explanation of Solution
Considering the bromination and chlorination of an alkane to check the
In the propagation process, the free radical of the reagent in step by step reacts with the
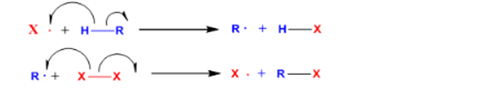
The initial stage controls the generation of a specific kind of radical.
The reaction enthalpy of this stage of bromination is measured as
The reaction enthalpy of this stage of chlorination is measured as follows:
Hence, the chlorination reaction is exothermic whereas the stage for bromination reaction is endothermic.
Hammond’s postulate the transition state of a reaction and would be nearer to the reactant in case of an exothermic reaction.
In chlorination reaction, the reaction is exothermic and thus the transition state is closer to the alkane than the radical generated.
The radical construction depends on the statistical factor.
In bromination reaction the reaction is endothermic and hence the transition state is nearer to the radical generated than the alkane.
The radical formation depends on the stability of the radical and more selectivity is attained.
(b)
Interpretation: The reason behind the dangerousness of fluorination needs to be explained.
Concept Introduction : Hess's law states that when the reactants are converted to products the change of enthalpy (i.e. the heat of reaction at constant pressure) does not dependent on the pathway between the initial and final states.
Hess’s law states that enthalpy changes are additive. Thus, for a single reaction,
(b)
Answer to Problem 10E
High exothermic nature of the bond formation, a huge amount of heat is liberated during fluorination hence the fluorination is dangerous.
Explanation of Solution
The bond formation enthalpy of carbon fluorine bond formation is as follows:
Due to this high exothermic nature of the bond formation, huge amount of heat is liberated during fluorination. Thus, the process is dangerous.
(C)
Interpretation: The reason behind the difficulty in the generation of an alkyl iodide by free radical chain halogenations needs to be explained.
Concept Introduction : Hess's law states that when the reactants are converted to products the change of enthalpy (i.e. the heat of reaction at constant pressure) does not dependent on the pathway between the initial and final states.
Hess’s law states that enthalpy changes are additive. Thus, for a single reaction,
(C)
Explanation of Solution
The iodination reaction of an alkane can be expressed as follows
The enthalpy change in this reaction is calculated as
As the reaction is endothermic in nature the reaction is difficult to carry out at room temperature.
The reverse reaction can also occur which further decreases the yield of the reaction.
Want to see more full solutions like this?
Chapter 9 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
- a. How many monochlorination products can be obtained from the radical chlorination of methylcyclohexane? Disregard stereoisomers.b. Which product would be obtained in greatest yield? Explain.c. How many monochlorination products would be obtained if all stereoisomers are included?arrow_forwardWhat alkene would you start with if you wanted to synthesizea. pentane? b. ethylcyclopentane?arrow_forwardMarkovnikov's rule can best be explained by considering; A. the number of hydrogen atoms attached to each of the double bond carbons atoms. B. the number of alkyl groups attached to each of the double bond carbons atoms. C. the spatial arrangement of the groups around the double bond. D. the relative stabilities of the carbocations that can be formed from each of the double bond carbon atoms.arrow_forward
- 1. 2-bromobutane + cyclohexanol + NaH à (major product) c. 2-butoxycyclohexane a.. 2-butene reaction b. 1-butene d. no e. something else! 2. t-butylbromide + sodium ethoxide in ethanol à (major product) a. 2-methylpropene reaction b. t-butyl ethyl ether c. 1-methylbutene d. no e. something else! 3. potassium t-butoxide + 1-bromobutane in t-butyl alcohol room temperature à (major product) a. 1-butene reaction c. 2-methylpropene d. no b. butyl t-butyl ether e. something else! 4. t-butyl bromide + boiling hot water à (major product) a. 2-methylpropene reaction b. t-butyl ethyl ether c. t-butanol d. no e. something else! 5. 2-chloropropane + acetic acid (2 eq) / KOH (1 eq) / DMF à(major product) c. b. d. no reaction e. something а. HO. else! Br KOH / DMSO 6. b. 2-methyl-1-propanol d. 1,2- a. 2-methylpropene propanediene c. no reaction e. something else! 7. cyclopentanol+ NaH + DMSO + bromopropane à(major product) a. cyclopentene reaction b. propene e. something else!! c. propyl cyclopentyl…arrow_forward2. For each of the following molecules, draw all possible mono-brominated products of an allylic bromination. (hint: Don't forget to look for resonance forms of the allylic radical initially formed.) a. b. f aarrow_forward1. Draw the structure of m-nitrotoluene using Chemsketch. 2. Name and Draw the structures of all possible chemical (Electrophilic aromatic substitution) reactions of the m-nitrotoluene;a. Halogenation (Chlorination or Bromination)b. Nitrationc. Sulphonationd. Friedal Craft Alkylatione. Friedal Craft Acylationarrow_forward
- [Review Topics] [References] Use the References to access important values if needed for this question. Draw the structural formula of the product that would form when 3-isopropylcyclopentene undergoes catalytic hydrogenation. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. C P. opy aste ChemDoodle Submit Answer Retry Entire Group 4 more group attempts remaining Previousarrow_forward6. Consider the following reaction: CH3 HBr. ? ČH3 a) Draw the two possible allylic cations that could be produced as an intermediate in this addition reaction; include resonance structures. b) Which of these allylic cations is more stable, explain briefly how you arrived at that conclusion?arrow_forwardDraw the structure of the major organic product of the reaction. 1. LIAIH4, ether 2. H₂0 ● You do not have to consider stereochemistry. Submit Answer ***I Q CI / Retry Entire Group [ ] در ? ChemDoodleⓇ [F 3 more group attempts remaining view Topics] [References]arrow_forward
- 1. i.What are the various ways by which alkenes may be synthesized? ii. Give two examples each of Unsymmetrical alkenes and reagents. iii. Give two examples of reactions of alkenes that result in Anti-Markonikov’s addition productsarrow_forwardA reaction flask contains 2-bromopentane in an ethanolic solution of sodium ethoxide at room temperature and result in the formation of two olefnic products. What is responsible for the formation of major and minor products. A. Different activated complex involved in the mechanism. B. Bimolecular nucleophilic substitution reaction. C. Bimolecular elimination reaction D. The presence of sodium ethoxide. E. The hybridization nature of secondary carbocationarrow_forward3. Explain in terms of bonding why ethene mostly undergoes electrophilic addition reactions. Draw the mechanism for the reaction of but-1-ene and hydrogen bromide. Show, via the mechanisms that are two different possible isomers – explain which one is formed preferentially and why this occurs.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





