
Concept explainers
(a)
Interpretation: The characteristics of a molecule to act as a good initiator needs to be explained.
Concept Introduction: The initiator is used in the initiation step and it is used to start a
(b)
Interpretation: The reason for the unnecessary use of stoichiometric amount of the initiator needs to be explained.
Concept Introduction: Only a catalytic amount of initiator is required for initiation of reaction, further to which there is no role for the initiator in the chemical reaction.
(c)
Interpretation: For the given radical initiators, the bond homolysis needs to be shown using the curved arrow when they are heated or irradiated.
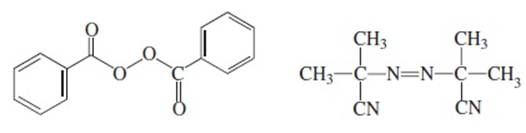
Benzoyl peroxide 2,2'-Azobis
Concept Introduction: In the bond dissociation, the movement of electrons always takes place from negative to positive charge. Thus, the direction of the curved arrow is also from negative to the positive charge.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
- 8. A student was asked to find the acid and basic radicals in six simple salts provided to him labeled as A,B,C,D,E and F. When he examined them, he observed that sample B was blue in colour and the remaining five salts were white. Answer the following questions. a. Why is concentrated HCl used in performing the flame test and not any other concentrated acid? b. What clue does this blue colour give about sample B? c. What is the importance of a negative test in salt analysis? d. The student is aware that he has to classify the anions into groups. What is the basis of this classification? e. Describe one test which distinguishes a bromide ion from a nitrate ion. f. The student observed that when dil HCl was added to a pinch of sample A. it gave brisk effervescence which turned lime water milky, while sample E gave a gas with rotten egg smell with the same reagent. Identify the acid radicals in sample A and E from the above observation and write the balanced equations involvedarrow_forwardWhich is effective method to determine OH. radicals in photoelectrocatalytic reaction?arrow_forwardNitric oxide, NO(g), is reduced at 550 K on rhodium catalyst through reaction with hydrogen, H2(g). Hydrogen dissociates when adsorbed on the catalyst while NO is strongly adsorbed. a. Propose a mechanism for the reaction by assuming it follows Langmuir-Hinshelwood kinetics. b. Derive the rate law for the reaction.arrow_forward
- 2 Provide the set of conditions to yield the following molecule. CH3 A. 1. NANH2, NH3, 2. CH,I 3. O, 4. Zn, H,0 B. 1. NaNH2, NH3, 2. CH3I 3. H2SO4, H20, Hg* C. H2SO, H20, Hg2 D. 1. H2, Pd, Lindar's catalyst 2. H3C D B. OO OOarrow_forward15. a. Label the reactive features of the following reactants, select the most reactive feature, then write and highlight what it needs. Also, state if a carbocation, carbon radical, or carbanion will start to develop, and/or if aromatic character will be lost as a result of a reaction between these molecules. p-fluorotoluene + Cl2 and FeCls b. Use mechanism arrows to illustrate the reaction that occurs. If applicable, use stabilization resources to deal with the carbocation, carbon radical, or carbanion that starts to develop during the reaction, and draw the structure of any resonance- stabilized intermediate. Continue labelling and diagramming the reaction until you find the major stable product(s). Finally, state the stereochemistry of the major product(s) and use either Fisher projection or perspective formula representations to illustrate that stereochemistry.arrow_forwardWhat term most accurately describes the process below? a. hydrogen abstraction b. halogen abstraction c. initiation d. couplingarrow_forward
- Consider the reaction of two compounds ‘A’ and ‘B’ which could make two possible diastereomers ‘AB’ and ‘BA’ (much like this week’s Diels Alder reaction). Hand-write your calculations and responses to the following questions and upload your work as a .jpg or .pdf file. Which of the two products (A or B) will form in greater abundance under thermodynamic control? Which will form in greater abundance under kinetic control? Explain your responses using a sketch of the reaction coordinate diagram for the reactions described above.arrow_forward7. Why is water a suitable solvent for this recrystallisation? 8. Calculate the concentration in mol dm-3 of 10 w/v% sodium hydroxide. 9. Write down an organic chemistry mechanism (use curly arrows) for the transformation of benzamide to benzoic acid, via its sodium salt.arrow_forwardWhat would the orientation factor be for this reaction? Why? (>1, <1, or =1)arrow_forward
- Give two (2) examples of reactions for these of the types of organic reactions: addition and rearrangement. Show: a. the overall reaction (reactants --> products) b. the reaction mechanism (indicate intermediate product) c. indicate which is the reactive species or intermediate in the reaction (radical? electrophile? nucleophile?) d. overall description of the reaction eg., radical substitution or SRarrow_forwardProfessor Lolita Carbon has assigned the analysis of unknown hydrocarbon samples to Enteng Mabutingting based on their reaction profile. The summary of the observed reactions are tabulated below along with the possible structures of the samples. Identify each unknown sample by matching the respective reaction profile with the given structures. Name each structure then briefly explain the reason for your answer. Sample Br, in CCI, (dark) Br, in CCI, (light) KMno, in NaOH AGNO, in NH3 Fading of reddish- 1 No reaction No reaction No reaction brown color Fading of reddish-brown Fading of reddish- Brown precipitates No reaction color brown color Fading of reddish-brown Fading of reddish- 3 Brown precipitates Silver precipitates color brown color H3C. CH3 CH3 CH3 H3C H3C CH3 H3C Sample Structure (3 pts) Name (2 pts) Rationalization (2 pts) 1 2 3arrow_forwardWhich of the statement is INCORRECT? a. The increase in stability of 2,4-hexadiene over 1,3-hexadiene is due to the increased double bond substitution of the former. b. The stabilization of dienes by conjugation is less pronounced than the aromatic stabilization of benzene. c. Resonance description in alkenes usually involves charge separation. d. Higher energy pi-orbitals often have decreasing number of nodes.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





