
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
6th Edition
ISBN: 9781305687875
Author: Gilbert
Publisher: CENGAGE LEARNING - CONSIGNMENT
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 9.2, Problem 15E
Interpretation Introduction
Interpretation: The relative reactivity of the H atoms in 1-chlorobutane needs to be determined depending on the percentage calculation of dichlorobutane formed in the reaction.
Concept introduction: 1-Chlorobutane has a chemical formula as
Following are the isomeric products formed when a 1-chlorobutane is monochlorinated using sulfuryl chloride with ABCN as the catalytic agent:
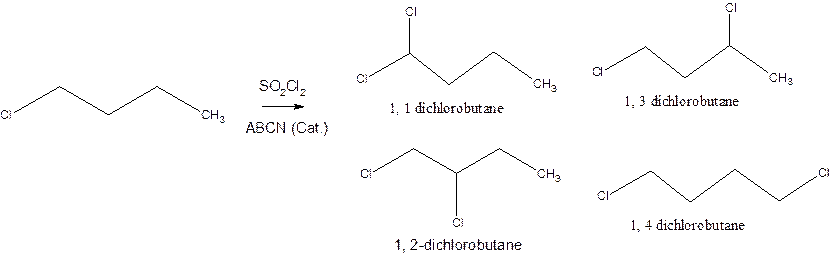
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Calculate the theoretical yield and percent yield of the product formed from.
Reaction between 1.005g Tetraphenylcyclopentadienone and 1.203 Maleic Anhydride with 15mL of xylene
The yield obtained was 1.097g.
What is the name of the product?
What two stereoisomeric alkanes are formed in the catalytic hydrogenation of (Z)-3-methyl-2-hexene? Draw and label the alkanes. In this reaction, what are the relative amounts of each of the two alkanes generated?
Free-radical chlorination of hexane gives very poor yields of 1-chlorohexane, while cyclohexane can be converted to chlorocyclohexane in good yield. What ratio of reactants (cyclohexane and chlorine) would you use for the synthesis of chlorocyclohexane?
Chapter 9 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
Ch. 9.2 - Prob. 1ECh. 9.2 - Prob. 2ECh. 9.2 - Prob. 3ECh. 9.2 - Prob. 4ECh. 9.2 - Prob. 5ECh. 9.2 - Prob. 6ECh. 9.2 - Prob. 7ECh. 9.2 - Prob. 8ECh. 9.2 - Prob. 9ECh. 9.2 - Prob. 10E
Ch. 9.2 - Prob. 11ECh. 9.2 - Prob. 12ECh. 9.2 - Prob. 13ECh. 9.2 - Prob. 14ECh. 9.2 - Prob. 15ECh. 9.2 - Prob. 16ECh. 9.2 - Prob. 17ECh. 9.2 - Prob. 18ECh. 9.2 - Prob. 19ECh. 9.2 - Prob. 20ECh. 9.2 - Prob. 21ECh. 9.2 - Prob. 22ECh. 9.2 - Prob. 23ECh. 9.2 - Prob. 24ECh. 9.3 - Prob. 1ECh. 9.3 - Prob. 2ECh. 9.3 - Prob. 3ECh. 9.3 - Prob. 4ECh. 9.3 - Prob. 5ECh. 9.3 - Prob. 6ECh. 9.3 - Prob. 7ECh. 9.3 - Prob. 8ECh. 9.3 - Prob. 9ECh. 9.3 - Prob. 10ECh. 9.3 - Prob. 11ECh. 9.3 - Prob. 12ECh. 9.3 - Prob. 13ECh. 9.3 - Prob. 14E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- G.170.arrow_forwardFree-radical chlorination of hexane gives very poor yields of 1-chlorohexane, while cyclohexane can be converted to chlorocyclohexane in good yield. How do you account for this difference?arrow_forwardUsing bond-line form with dash-wedge (perspective formulas) draw the structure of both the R and S stereocenters for the organic products and give the corresponding IUPAC names, including their stereochemistry, of the following reactions. Write your answer in the blank spacearrow_forward
- [Review Topics] [References] Use the References to access important values if needed for this question. Draw the product that would form when 3,3-dimethyl-1-butene reacts with chlorine. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. . In cases where there is more than one answer, just draw one. Sn [F ChemDoodle An error has been detected in your answer. Check for typos, miscalculations etc. before submitting your answer. C Draw the product that would fa Previousarrow_forwardThis it thermo chemistry q , kindly solve with all detailes as soon as possible , thank youarrow_forwarda) Predict the major product of the reaction and identify the type of reaction (substitution or addition). Explain the function of Pt used in the reaction. (with First image) b) Write the reaction of the mononitration of chlorobenzene and predict the major products with reasons. c) Identify all the carbon atoms in the following compounds that are sp2 (Circle the carbon(s) your selected on image two)arrow_forward
- Please anarrow_forward1-pentanol + NaBr/H2SO4 -> 1-bromopentane Is this substitution reaction reversible? In other words, would one expect to obtain an equilibrium mixture of the alcohol and bromoalkene at the end of the reaction of would one expect this reaction to go to completion? Justify your answer?arrow_forwardBromine in methylene chloride is added in stoichiometric proportions to cyclopentane, What color is the final solution? Bromine in methylene chloride is added in stoichiometric proportion to cyclohexa-1,3-diene. What color is the final solution?arrow_forward
- Draw the alkene that would react with the reagent given to account for the product formed. OH H₂SO4 CH3CH2CCH2CH3 ? + H₂O CH2CH3 . You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one.arrow_forwardBromine is a larger atom than chlorine, but the equilibrium constants in Table indicate that a chloro substituent has a greater preference for theequatorial position than does a bromo substituent. Suggest an explanation for this fact.arrow_forwardThe compound below is treated with chlorine in the presence of lightarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT