
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
a) Predict the major product of the reaction and identify the type of reaction (substitution or addition). Explain the function of Pt used in the reaction. (with First image)
b) Write the reaction of the mononitration of chlorobenzene and predict the major products with reasons.
c) Identify all the carbon atoms in the following compounds that are sp2 (Circle the carbon(s) your selected on image two)
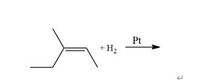
Transcribed Image Text:Pt
+ H2
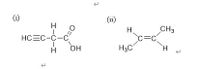
Transcribed Image Text:(i)
(11)
H.
CH3
HCEC-C-C
c=c
H3C
H.
HO
H-CIH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- e structures for the least and most polar molecules (i.e., isomers) of Bromo-chloro-flouro- raw iodoethene (C2BrCIFI). Briefly explain your choice. Structure of least polar: Structure of most polar:arrow_forwardDraw the contributing structure that results from resonance indicated by the curved arrow(s). You do not have to consider stereochemistry. Explicitly draw all H atoms. You do not have to include lone pairs in your answer.arrow_forwardOrganic Chemistry Question PLEASE SHOW STEP BY STEP, WITH ARROWS! ALSO PLEASE INCLUDE ALL ELECTRON LONE PAIRS AND CHARGES FOR STRUCTURES IF NECESSARY!arrow_forward
- is this z or E?? If it is E. please in details explain why cycloproyl is greater than OH group???arrow_forwardWhich c. ..se structures shows how the pictured molecule will look after it is flipped over horizontally, as illustrated? он flip OH H H. OH он H. ..l OH none of these но Note: Only check the boxes of structures that correspond to a single horizontal flip of the original structure. Ignore structures that have been rotated or flipped multiple times or in other ways.arrow_forward(a) In one of the two boxes below, draw a wedge and dashed wedge structure (picture) of CH3Cl that best illustrates the geometry about the central atom. In the other box, draw another picture of the model from a different angle (viewpoint). (b) In CH3Cl, are the three hydrogen atoms equivalent (i.e., do they have identical environments with respect to the other atoms adjacent to themselves)? Briefly explain the evidence for your answerarrow_forward
- Need to check answer 1.Borane (BH3) adds to alkenes to form an alkylborane. In the first box draw the mechanism arrows, and in the second box draw the correct product. Be sure to add lone pairs of electrons and nonzero formal charges to all species.arrow_forwardProblem (#2.) For each ion below, draw all reasonable resonance structures (linked by resonance arrows “↔”). Include curved arrows that indicate the movement of electrons between each resonance structure. Assign non-zero formal charges to each atom for each resonance structure. (a.) NO3– (nitrate) (b.) CH3COO– (acetate) (c.) N3– (azide) (d.) NCO– (isocyanate) Problem (#3.) For each ion in question 2, draw a resonance hybrid, assigning non-zero formal and/or partial charges (δ+, δ–) as needed. Problem (#4.) For each skeletal structure below, satisfy the valences (or octets) of all of the atoms by filling in double and triple bonds as well as unshared electron pairs. Assign non-zero formal charges and show the overall charge if the structure is an ion. See photo attached for Problem number 4. Problem (#5.) For each structure in question 4, draw a resonance hybrid (if it has one) and assign non-zero formal and/or partial charges as needed.arrow_forwardadd one double‑barbed curved arrow to each structure to show the delocalization of the electron pair to form the next structurearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY