
Concept explainers
Interpretation:
Among the given physiologically active
Concept Introduction:
Generally amines are toxic in nature. If a compound contains only amine as its
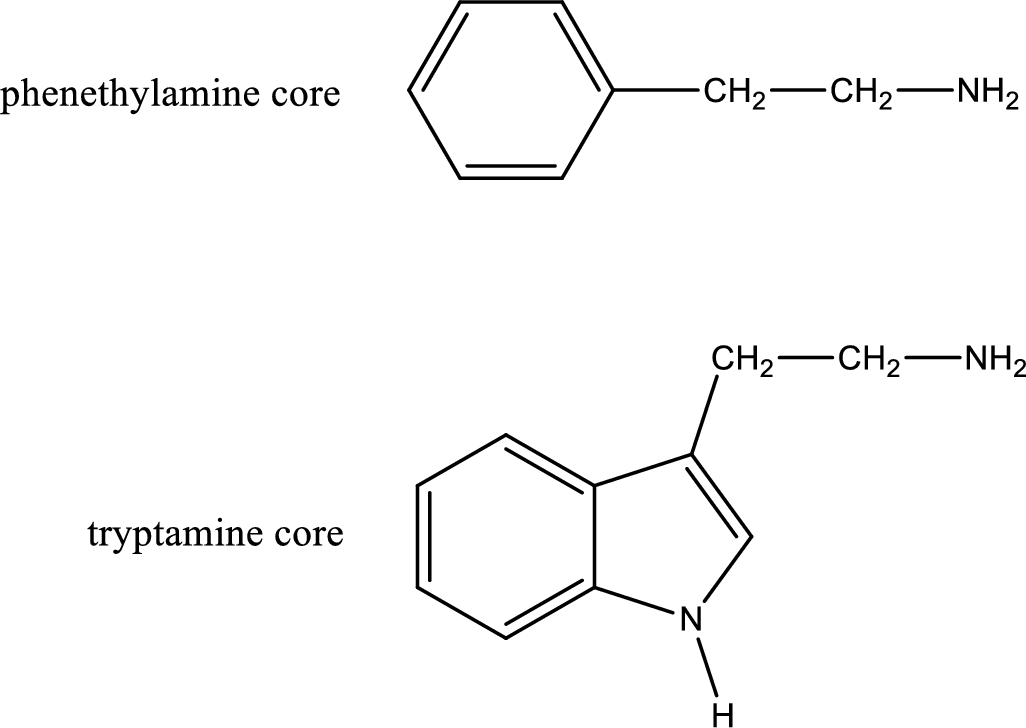
There are three types of effects that an amine can exert. They are neurotransmitters, central nervous system stimulants and decongestants. Neurotransmitters are the substances that are present in human body that help in passing impulse of nerves from one cell to another. Central nervous stimulants are the substances that help in speeding up of physical and mental processes. Decongestant is a substance that is used to relieve nasal congestion.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Organic And Biological Chemistry
- CHE102 B urse Home KCHE102 Trad Exam 1 Item 6 Part A What is the mass of 3.00 L of an intravenous glucose solution with a density of 1.25 g/ml? Submit Request Answer Provide Feedback Marrow_forwardThe word amino would be present in the IUPAC name of which of the following compounds? CH3-CH-CH2-CH3 NH₂ CH3-CH-CH₂-OH I NH₂ CH3-CH–CH2-C1 NH₂ more than one correct response no correct response Previous Page Next Page Page 13 of 16arrow_forwardWhat is the [HA] (in mol L-1) of a solution containing 1.074 mol L-1 of a diprotic acid with pKA1 = 3.29 and pKA2 = 9.5 ? H2A + H2O = H30t + HA pkA1 HA + H20 = H3O+ + A2- pka2 Answer:arrow_forward
- Which of the following sets of reactants could be used to produce an amine salt? ammonia and hydrochloric acid diethylammonium chloride and sodium hydroxide O methylamine and water O no correct responsearrow_forwardWhat two chemicals can be reacted together to fom an ester? A- An alcohol and a carboxylic acid B- A carboxylic acid and an alkali C- Two carboxylic acids D-Non of these Answer A Answer B Answer C Answer Darrow_forwardAnswer 1 2 3 4arrow_forward
- When the head and two tails structural model is applied to a glycerophospholipid, the two tails are a. both fatty acid residues b. a fatty acid residue and a phosphate group c. the phosphate group and the glycerol backbone d. no correct responsearrow_forwardWhat is the [HA¯] (in mol L-¹) of a solution containing 0.846 mol L-1 of a diprotic acid with pKÅ₁ = 4.82 and PKA2 = 9.19? Answer: H2A + H2OH3O++ HA¯ PKA1 HA¯ + H2O = H3O++ A²- PKA2arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,

