
Concept explainers
(a)
Interpretation:
What amide
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Organic compounds contain heteroatom also. Some of them are nitrogen, sulfur, oxygen etc. Nitrogen containing organic compounds are of two important types and they are
One of the
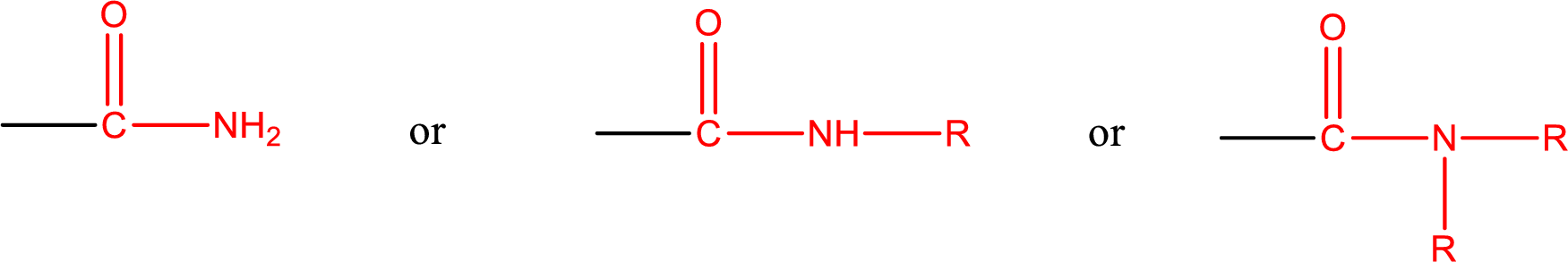
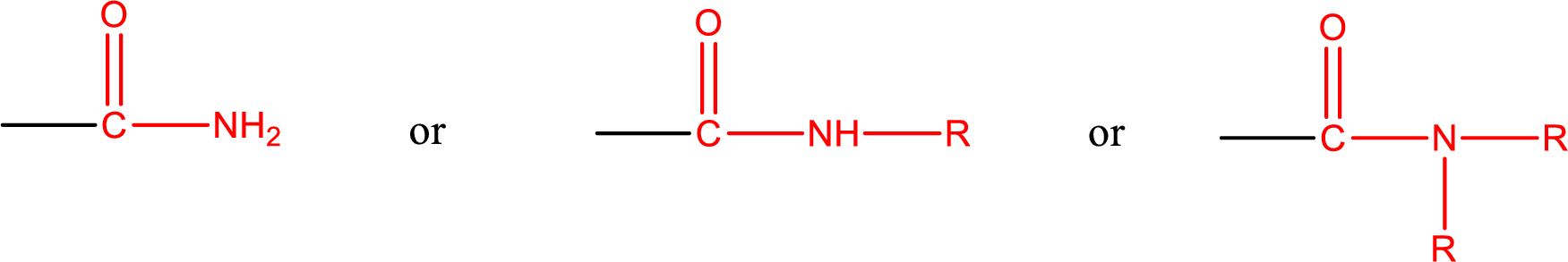
Amides are also classified as primary, secondary, and tertiary amide.
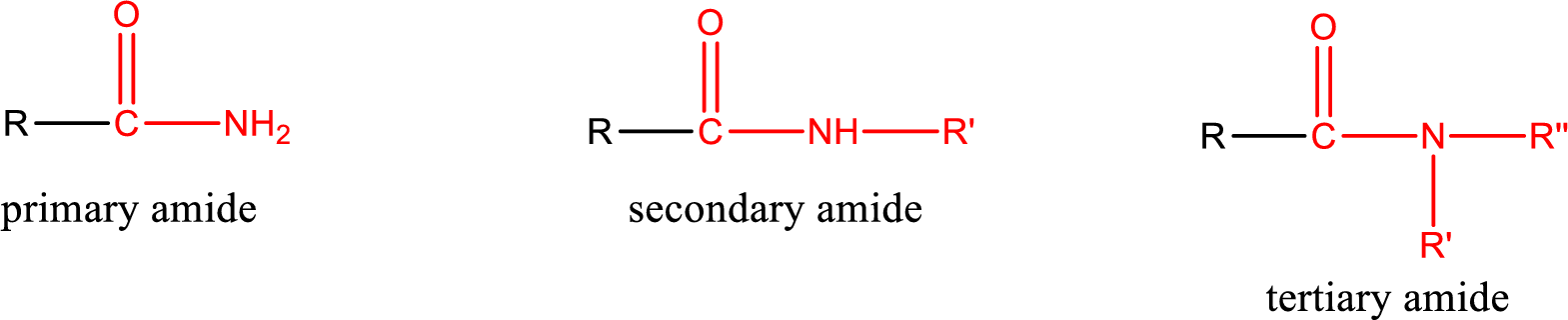
Primary amide is the one that has a nitrogen atom that is bonded to two hydrogen atoms. Primary amides are also known as unsubstituted amides.
Secondary amide is the one that has a nitrogen atom that is bonded to one hydrogen atom and one alkyl (or aryl) group. Secondary amides are also known as monosubstituted amides.
Tertiary amide is the one that has a nitrogen atom that is bonded to two alkyl (or aryl) groups. Tertiary amides are also known as disubstituted amides.
(b)
Interpretation:
What amide functional group that the given compound contains has to be indicated.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Organic compounds contain heteroatom also. Some of them are nitrogen, sulfur, oxygen etc. Nitrogen containing organic compounds are of two important types and they are amines, amides.
One of the carboxylic acid derivative is amide. In this the carboxyl
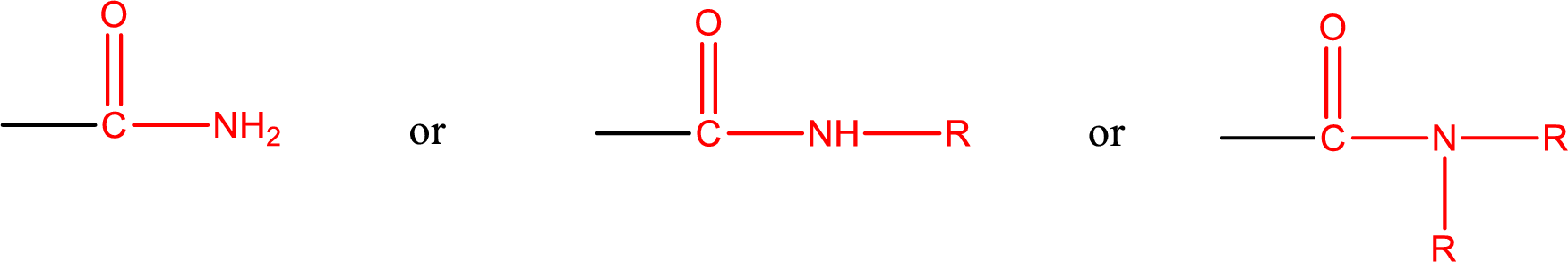
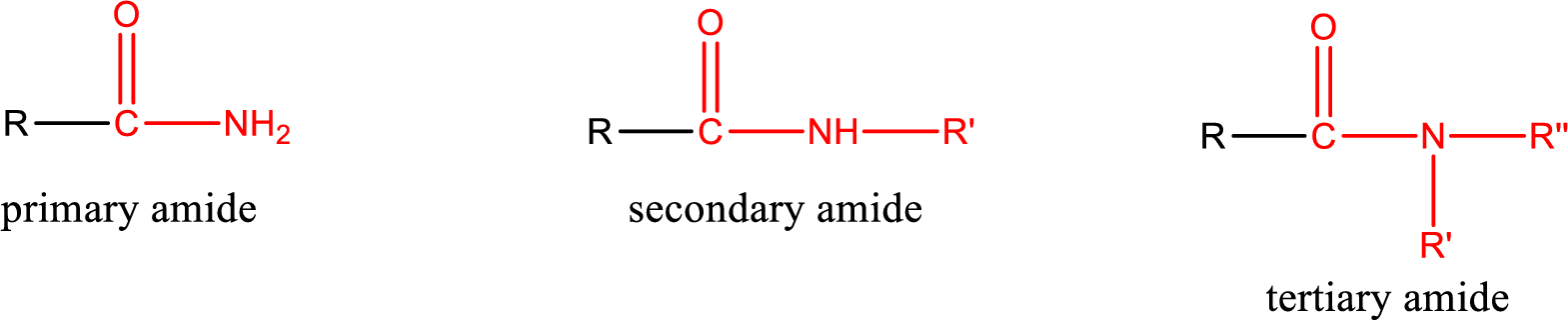
Amides are also classified as primary, secondary, and tertiary amide.
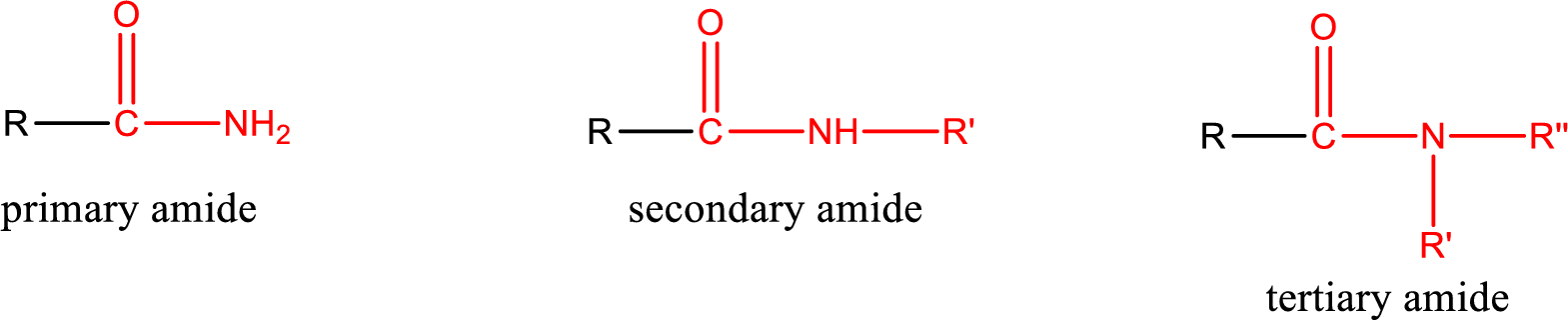
Primary amide is the one that has a nitrogen atom that is bonded to two hydrogen atoms. Primary amides are also known as unsubstituted amides.
Secondary amide is the one that has a nitrogen atom that is bonded to one hydrogen atom and one alkyl (or aryl) group. Secondary amides are also known as monosubstituted amides.
Tertiary amide is the one that has a nitrogen atom that is bonded to two alkyl (or aryl) groups. Tertiary amides are also known as disubstituted amides.
(c)
Interpretation:
What amide functional group that the given compound contains has to be indicated.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Organic compounds contain heteroatom also. Some of them are nitrogen, sulfur, oxygen etc. Nitrogen containing organic compounds are of two important types and they are amines, amides.
One of the carboxylic acid derivative is amide. In this the carboxyl
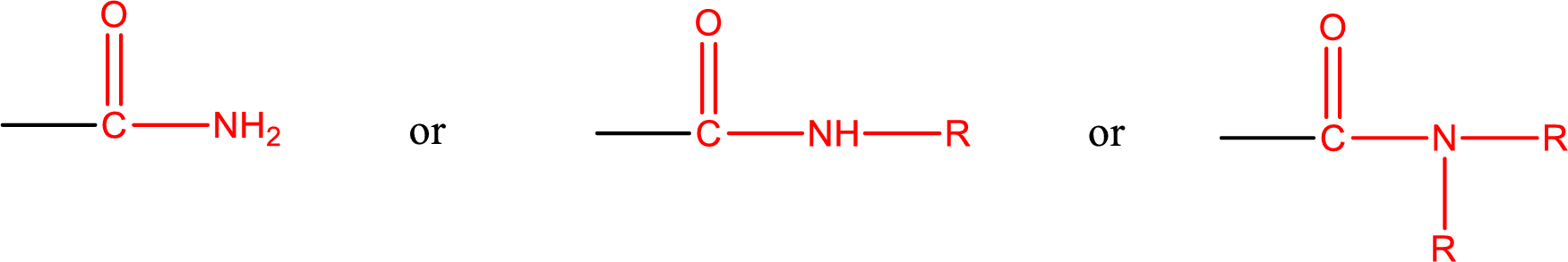
Amides are also classified as primary, secondary, and tertiary amide.
Primary amide is the one that has a nitrogen atom that is bonded to two hydrogen atoms. Primary amides are also known as unsubstituted amides.
Secondary amide is the one that has a nitrogen atom that is bonded to one hydrogen atom and one alkyl (or aryl) group. Secondary amides are also known as monosubstituted amides.
Tertiary amide is the one that has a nitrogen atom that is bonded to two alkyl (or aryl) groups. Tertiary amides are also known as disubstituted amides.
(d)
Interpretation:
What amide functional group that the given compound contains has to be indicated.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Organic compounds contain heteroatom also. Some of them are nitrogen, sulfur, oxygen etc. Nitrogen containing organic compounds are of two important types and they are amines, amides.
One of the carboxylic acid derivative is amide. In this the carboxyl
Amides are also classified as primary, secondary, and tertiary amide.
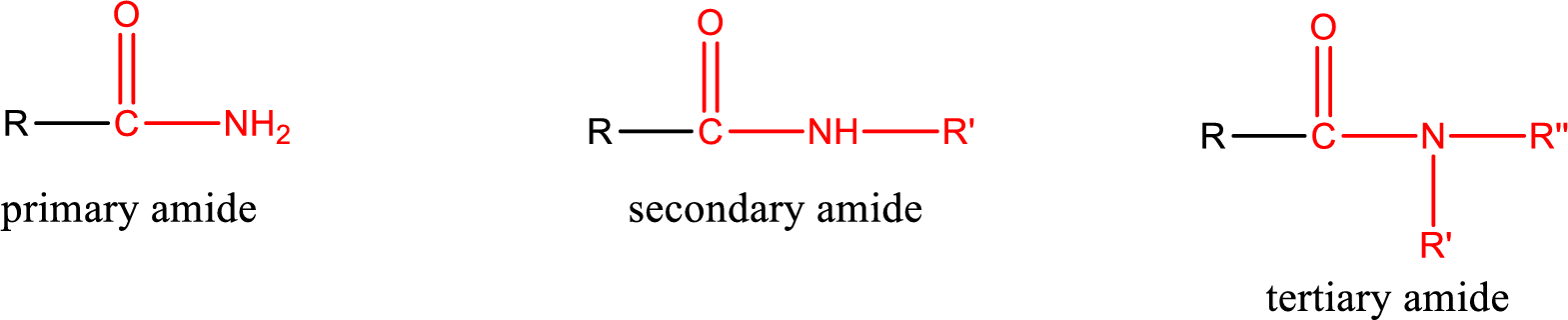
Primary amide is the one that has a nitrogen atom that is bonded to two hydrogen atoms. Primary amides are also known as unsubstituted amides.
Secondary amide is the one that has a nitrogen atom that is bonded to one hydrogen atom and one alkyl (or aryl) group. Secondary amides are also known as monosubstituted amides.
Tertiary amide is the one that has a nitrogen atom that is bonded to two alkyl (or aryl) groups. Tertiary amides are also known as disubstituted amides.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Organic And Biological Chemistry
- Indicate whether or not each of the following compounds contains an amine functional group?arrow_forwarddraw the condensed structural formula for the product from the hydrolysis of each of the following amides with HClarrow_forwardAccount for the fact that most low-molecular-weight amines are very soluble in water, whereas low- molecular-weight hydrocarbons are not.arrow_forward
- Please draw a hydrogen bond between an amine group and a carboxyl group. Use a dotted line to indicate the hydrogen bond itself. Label the hydrogen bond donor and the hydrogen bond acceptor.arrow_forwardComplete this table for different amine compounds. Chemical propylamine quaternary ammonium ion methylphenylamine Molecular formula C3H9N C₂H7N C5H13N C4H10N Structural formula (CH3)3N (CH3)2NH CH 3 I (CH₂) 11 | H3C(CH2) 11 -N-(CH₂)11CH3 NHCH3 (CH₂)11 CH3 CH3CH2CH2-NH-CH3 + Classification Tertiary Quaternary Tertiary Secondaryarrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


