
Concept explainers
(a)
Interpretation:
The number of carbon‑nitrogen bonds present in primary amine has to be given.
Concept Introduction:
Amine is an organic derivative. If in ammonia one or more alkyl, cycloalkyl, or aryl groups are substituted instead of hydrogen atom then it is known as amine. Depending on the number of substitution the
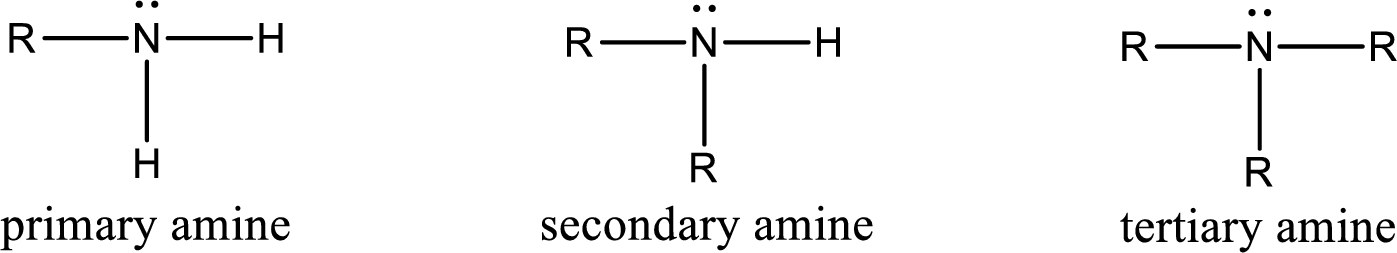
(b)
Interpretation:
The number of carbon‑nitrogen bonds present in secondary amine has to be given.
Concept Introduction:
Amine is an organic derivative. If in ammonia one or more alkyl, cycloalkyl, or aryl groups are substituted instead of hydrogen atom then it is known as amine. Depending on the number of substitution the amines are classified as primary, secondary or tertiary amine. Primary amine is the one in which only one hydrogen atom in ammonia is replaced by a hydrocarbon group. Secondary amine is the one in which only two hydrogen atoms in ammonia is replaced by a hydrocarbon group. Tertiary amine is the one in which all three hydrogen atoms in ammonia is replaced by a hydrocarbon group. The generalized structural formula for all the amines is,
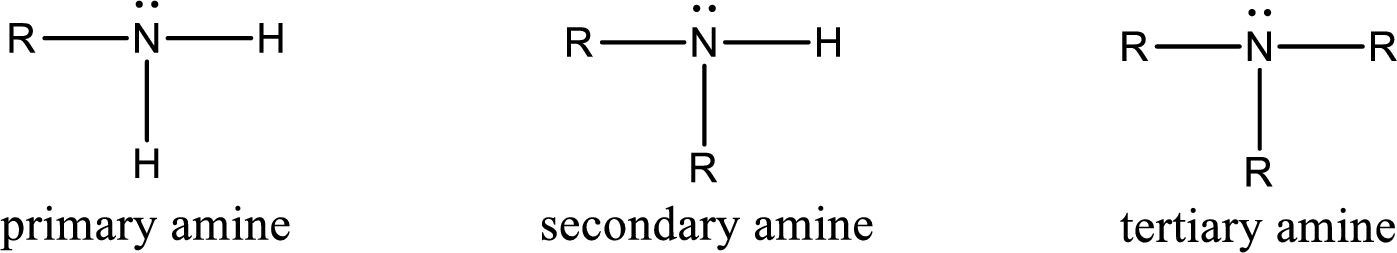
(c)
Interpretation:
The number of carbon‑nitrogen bonds present in tertiary amine has to be given.
Concept Introduction:
Amine is an organic derivative. If in ammonia one or more alkyl, cycloalkyl, or aryl groups are substituted instead of hydrogen atom then it is known as amine. Depending on the number of substitution the amines are classified as primary, secondary or tertiary amine. Primary amine is the one in which only one hydrogen atom in ammonia is replaced by a hydrocarbon group. Secondary amine is the one in which only two hydrogen atoms in ammonia is replaced by a hydrocarbon group. Tertiary amine is the one in which all three hydrogen atoms in ammonia is replaced by a hydrocarbon group. The generalized structural formula for all the amines is,
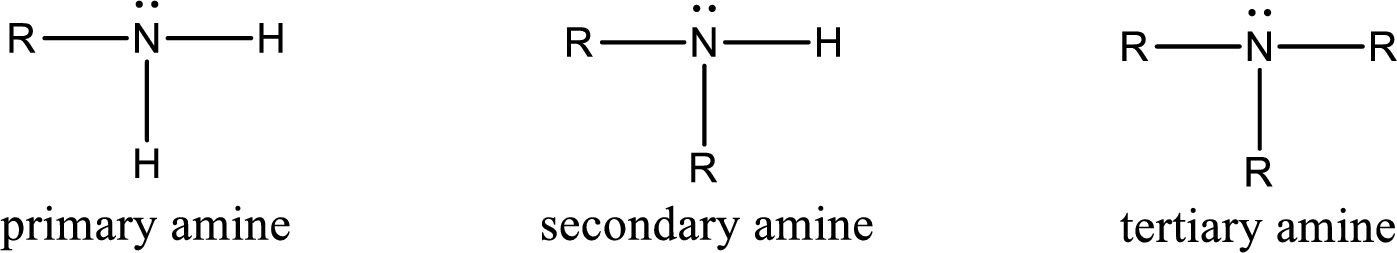
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Organic And Biological Chemistry
- a. 1° amine b. How many nitrogen-hydrogen bonds are present in the functional group in each of the following types of amines? a. 1° amine 17-6 b. 2° amine c. 3° amine Cthe followvingarrow_forwardThe hydrolysis of an amide in acidic conditions forms A. a carboxylate salt and an alcohol B. a carboxylate salt and an amine C. an alcohol and an amine salt (an ammonium ion) D. a carboxylic acid and an amine salt (an ammonium ion)arrow_forwardWhich of the following statements is true for an amine if "N-" is part of the IUPAC name? a. The compound is a primary amine. b. The molecule is contains a nitrogen atom attached to carbon number one. c. The compound is a secondary amine. d. The compound is a tertiary amine.arrow_forward
- How many carbon-nitrogen bonds are present in each of the following types of amines? a. primary amine b. secondary amine c. tertiary amine 17-4arrow_forward1. Draw the structure for each compound and classify the amine as primary, secondary, or tertiary. a. dimethylamine b. diethylmethylamine c. 2-aminoethanolarrow_forwardN-p-hydroxyphenylethanamide is commonly known as a. acetaminophen b. acetamide c. acetanilide d. formamide High molar mass amines have __________ odor. a.strong ammoniacal b.fruity c.fishy d.obnoxious Trimethyl amine has _________ odor. a.obnoxious b.fishy c. ammoniacal d. fruityarrow_forward
- Amide hydrolysis in basic conditions forms A. a carboxylic acid and an amine B. a carboxylate salt and an amine 3. an ester and an amine 4. a carboxylic acid and an amine saltarrow_forwardWhich statements are TRUE?I. Tertiary amines have lower BP than primary and secondary amines.II. Tertiary amines has no possibility for hydrogen bonding.III. Tertiary amines has a high-molecular mass as hydrogen bonding occursarrow_forwardCompound C4H11N is a a.secondary amine b.primary amine c.tertiary aminearrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





