
Concept explainers
(a)
Interpretation:
Structure of
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between
(a)
Answer to Problem 6.134EP
Carboxylic acid that is required was,
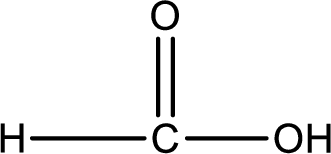
Explanation of Solution
Given structure of compound is,
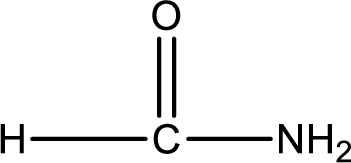
As the nitrogen atom present in the above amide has two hydrogen atoms bonded to it, the amide is a primary amide. Primary amide is produced by the reaction of ammonia with carboxylic acid. The “parent” carboxylic acid can be identified as shown below,
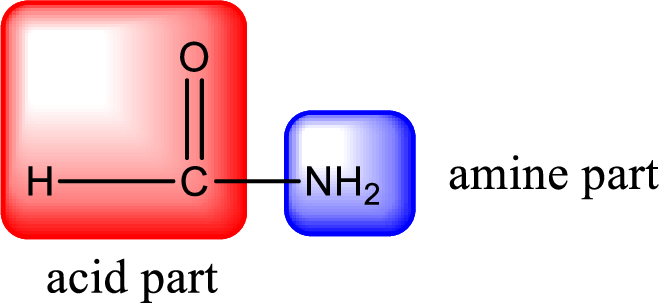
Hydrogen atom has to be added to the amine part and
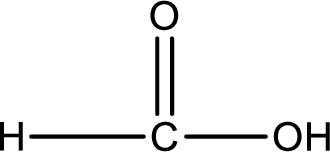
Structure of carboxylic acid that is required to produce the given compound is drawn.
(b)
Interpretation:
Structure of carboxylic acid that is required to produce the given compound as product through amidification has to be given.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between amine and carboxylic acid. In this reaction, the
(b)
Answer to Problem 6.134EP
Carboxylic acid that is required was,
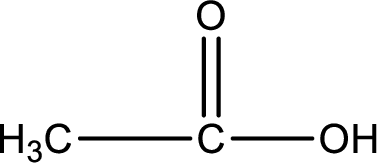
Explanation of Solution
Given structure of compound is,
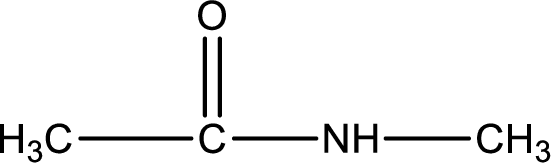
As the nitrogen atom present in the above amide has two hydrogen atoms bonded to it, the amide is a primary amide. Primary amide is produced by the reaction of ammonia with carboxylic acid. The “parent” carboxylic acid can be identified as shown below,
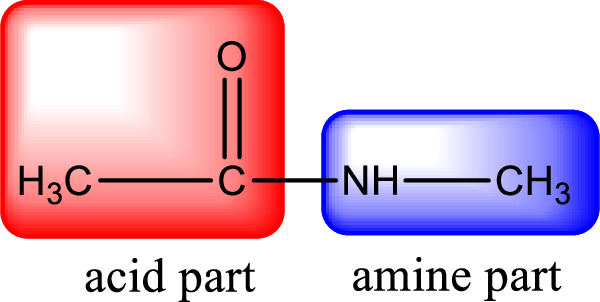
Hydrogen atom has to be added to the amine part and
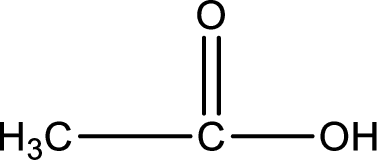
Structure of carboxylic acid that is required to produce the given compound is drawn.
(c)
Interpretation:
Structure of carboxylic acid that is required to produce the given compound as product through amidification has to be given.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between amine and carboxylic acid. In this reaction, the
(c)
Answer to Problem 6.134EP
Carboxylic acid that is required was,
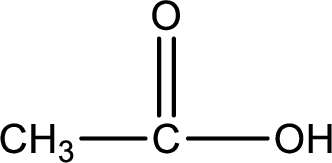
Explanation of Solution
Given structure of compound is,
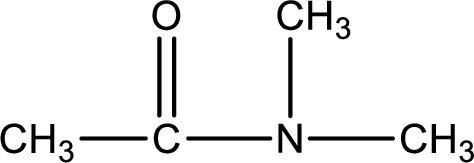
As the nitrogen atom present in the above amide has two hydrogen atoms bonded to it, the amide is a primary amide. Primary amide is produced by the reaction of ammonia with carboxylic acid. The “parent” carboxylic acid can be identified as shown below,
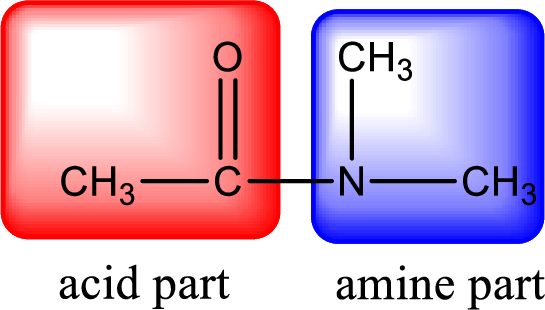
Hydrogen atom has to be added to the amine part and
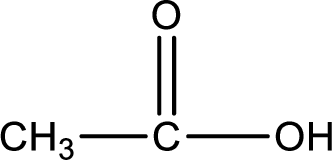
Structure of carboxylic acid that is required to produce the given compound is drawn.
(d)
Interpretation:
Structure of carboxylic acid that is required to produce the given compound as product through amidification has to be given.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between amine and carboxylic acid. In this reaction, the
(d)
Answer to Problem 6.134EP
Carboxylic acid that is required was,
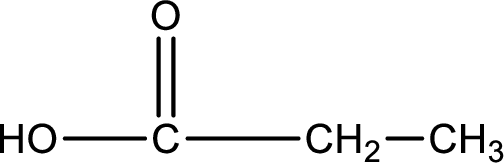
Explanation of Solution
Given structure of compound is,
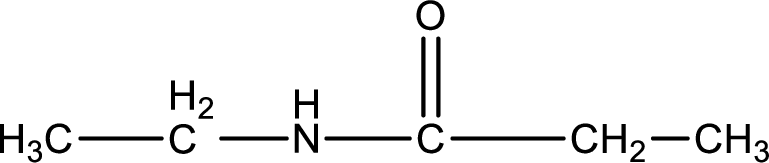
As the nitrogen atom present in the above amide has two hydrogen atoms bonded to it, the amide is a primary amide. Primary amide is produced by the reaction of ammonia with carboxylic acid. The “parent” carboxylic acid can be identified as shown below,
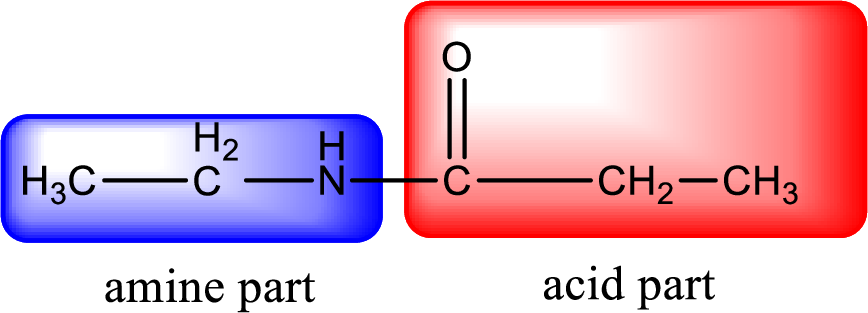
Hydrogen atom has to be added to the amine part and
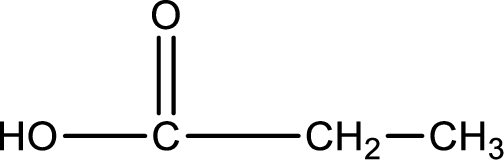
Structure of carboxylic acid that is required to produce the given compound is drawn.
Want to see more full solutions like this?
Chapter 6 Solutions
Organic And Biological Chemistry
- What is the class of organic compound that is produced when a carboxylic acid derivative undergoes hydrolysis? Carboxylic acid Alcohol Ester Acid Anhydridearrow_forwardThe presence of amides in living organisms is beneficial due its stability which results from being the least reactive carboxylic acid derivative. True or Falsearrow_forwardDraw the structure of the amide that can be made from the following reactants.arrow_forward
- Give the IUPAC name for the following acid amide: 0 CH3CH2CH(CH3)CH2CH(CH3)CH2C NH2arrow_forwardIndicate the category of base to which the following compound belongs: Сасоз Hydride Amine O Carbonate O Bicarbonate Hydroxide Alkoxide O Carbanion O Amidearrow_forwardWhat is the role of pyridine in the acylation reaction of amines?arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



