
Concept explainers
(a)
Interpretation:
The organic product formed in the given reaction has to be indicated as primary amide, secondary amide, tertiary amide, or not an amide.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between
(a)
Answer to Problem 6.132EP
Organic product obtained is a primary amide.
Explanation of Solution
Given reaction is,
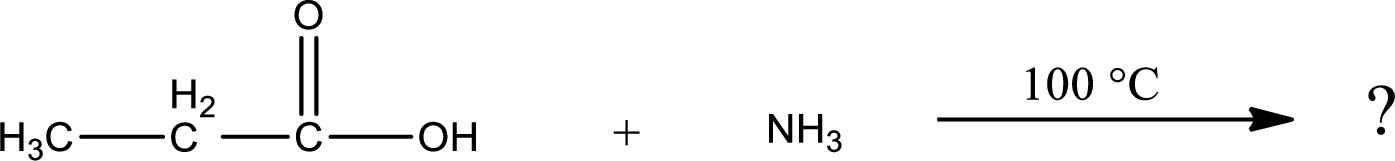
In amidification process,
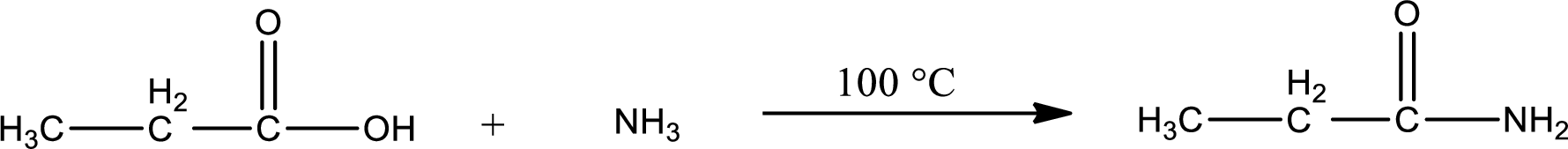
The product obtained is indicated.
(b)
Interpretation:
The organic product formed in the given reaction has to be indicated as primary amide, secondary amide, tertiary amide, or not an amide.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between amine and carboxylic acid. In this reaction, the
(b)
Answer to Problem 6.132EP
Organic product obtained is a secondary amide.
Explanation of Solution
Given reaction is,
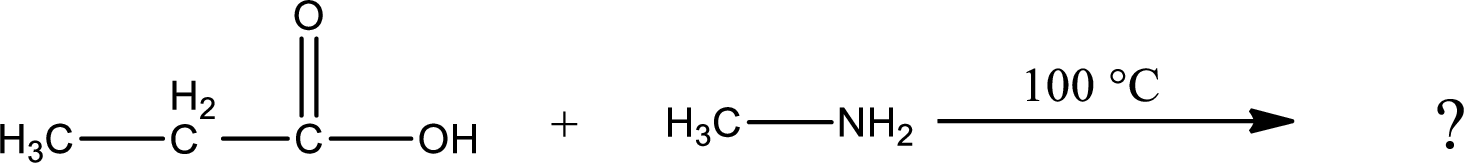
In amidification process,
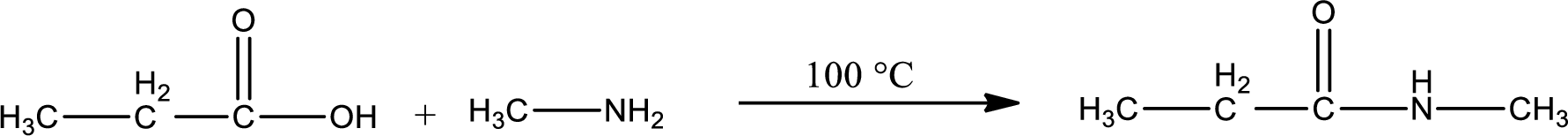
The product obtained is indicated.
(c)
Interpretation:
The organic product formed in the given reaction has to be indicated as primary amide, secondary amide, tertiary amide, or not an amide.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between amine and carboxylic acid. In this reaction, the
(c)
Answer to Problem 6.132EP
Organic product obtained is a tertiary amide.
Explanation of Solution
Given reaction is,
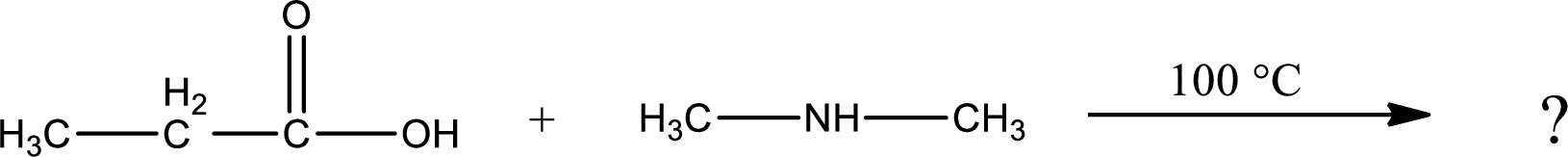
In amidification process,
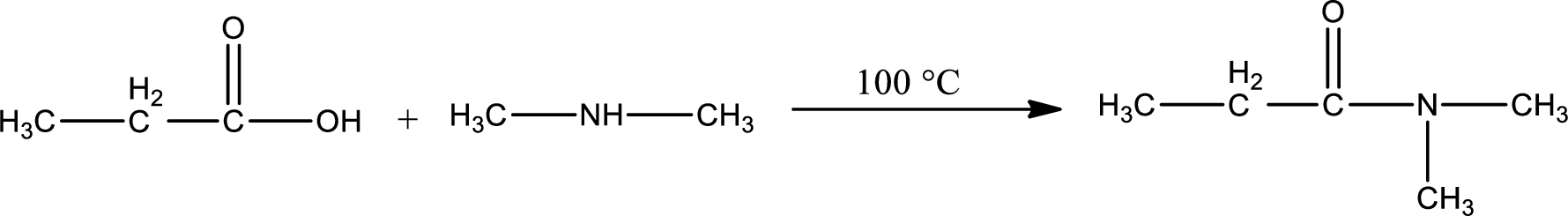
The product obtained is indicated.
(d)
Interpretation:
The organic product formed in the given reaction has to be indicated as primary amide, secondary amide, tertiary amide, or not an amide.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between amine and carboxylic acid. In this reaction, the
(d)
Answer to Problem 6.132EP
Organic product obtained is a tertiary amide.
Explanation of Solution
Given reaction is,

In amidification process,
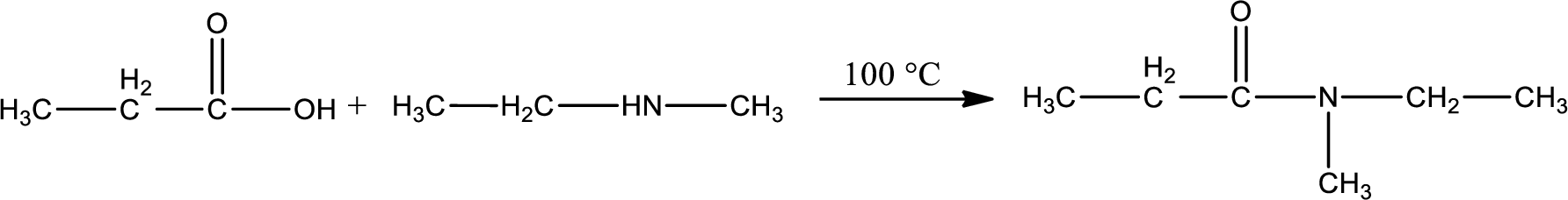
The product obtained is indicated.
Want to see more full solutions like this?
Chapter 6 Solutions
Organic And Biological Chemistry
- Complete this table for different amine compounds. Chemical propylamine quaternary ammonium ion methylphenylamine Molecular formula C3H9N C₂H7N C5H13N C4H10N Structural formula (CH3)3N (CH3)2NH CH 3 I (CH₂) 11 | H3C(CH2) 11 -N-(CH₂)11CH3 NHCH3 (CH₂)11 CH3 CH3CH2CH2-NH-CH3 + Classification Tertiary Quaternary Tertiary Secondaryarrow_forward< app.101edu.co Classify and describe the properties of the following nitrogen containing compound. O CI O + Question 13.a of 25 CH3 N Classify the following amine. H CH3 A) primary amine B) secondary amine C) tertiary amine @=J D) tertiary amine salt E) quaternary ammonium saltarrow_forwardBelow is the structure of Clemastine and its functional groups. Which of the following statements regarding Clemastine and its functional groups is NOT true? * Ether Tertiary amines CH CH3 Halogenated aromatic hydrocarbon Aromatic hydrocarbon Aliphatic hydrocarbons Clemastine (Tavist) Aromatic hydrocarbon is lipophilic Halogenated aromatic hydrocarbon is lipophilic. Tertiary amine is hydrophobic. Ether is hydrophilic.arrow_forward
- Which of the following amines has the LOWEST boiling point? ethylhexylamine triethylammonium chloride triethylamine non-1-aminearrow_forward17-52 Draw the structure of the amine produced when each of the following amine salts reacts with NaOH. a. CH3-CH2-CH2-NH, Cl- CH3 b. CH3-CH-NH2-CH3 Cl -NH, Cl" d. CH3 NH-CH, CI CH3arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



