
Becker's World of the Cell (9th Edition)
9th Edition
ISBN: 9780321934925
Author: Jeff Hardin, Gregory Paul Bertoni
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 6.5PS
Michaelis–Menten Kinetics. Figure 6-16 represents a Michaelis–Menten plot for a typical enzyme, with initial reaction velocity plotted as a function of substrate concentration. Three regions of the curve are identified by the letters A, B, and C. For each of the statements that follow, indicate with a single letter which one of the three regions of the curve fits the statement best. A given letter can be used more than once.
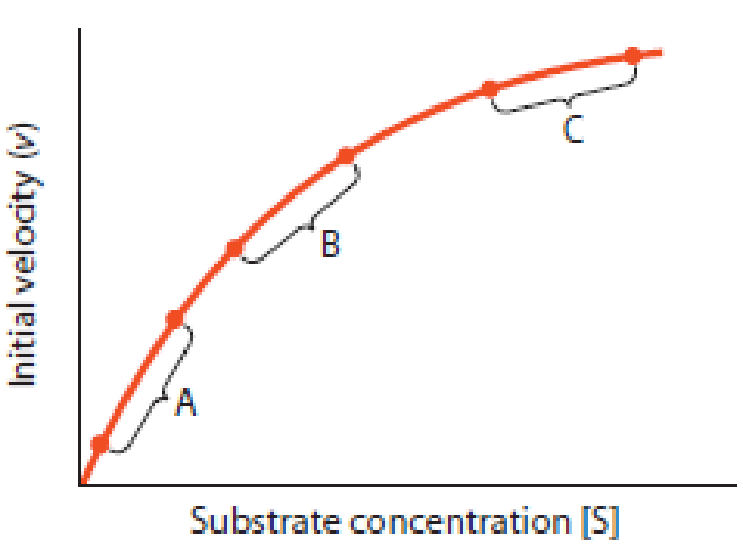
Figure 6-16 Analysis of the Michaelis–Menten Plot. See Problem 6-5.
- (a) The active site of an enzyme molecule is occupied by substrate most of the time.
- (b) The active site of an enzyme molecule is free most of the time.
- (c) This is the range of substrate concentration in which most enzymes usually function in normal cells.
- (d) This includes the point (Km, Vmax/2).
- (e) Reaction velocity is limited mainly by the number of enzyme molecules present.
- (f) Reaction velocity is limited mainly by the number of substrate molecules present.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
. Recall your study of equilibria and kinetics from general chemistry. You used equations with upper case
Kand lower case k during the study of equilibria and kinetics respectively. What do the upper and lower
case letters refer to?
Enzyme Action: Testing Catalase Activity
8) Explain why the [VO] of the reactions at pH 3 and temperature of 100'C were (or should have been)
tower than the other treatments conducted. In your response consider the following:
a) The effect of these conditions on the structure of the enzyme.
b) Why this structural effect resulted in a decreased [VO].
6-25
substrate-band enzyme concentrations. The the
turnover number is equal to umax-
b)
V=Umax •57(Km+S)
anstont
For an enzyme that displays Michaelis-Menten kinetics, what is
the reaction velocity, V (as a percentage of Vmax), observed at
the following values?
a)
[S] = KM
C)
d)
e)
[S] = 0.5KM
[S] = = 0.1KM
[S] = 2KM
[S] = 10KM
w
reactores
-maximumrate of reaction
boteles conc.
Would you expect the structure of a competitive inhibitor of a
given enzyme to be similar to that of its substrate?
Chapter 6 Solutions
Becker's World of the Cell (9th Edition)
Ch. 6 - Gasoline is highly combustible yet doesnt burst...Ch. 6 - How can an enzyme recognize and bind one specific...Ch. 6 - Prob. 1QCh. 6 - You work at a biotechnology company and are...Ch. 6 - Why do enzymes need to be regulated? By what...Ch. 6 - The Need for Enzymes. You should now be in a...Ch. 6 - Prob. 6.2PSCh. 6 - Prob. 6.3PSCh. 6 - Temperature and pH Effects. Figure 6-4 illustrates...Ch. 6 - MichaelisMenten Kinetics. Figure 6-16 represents a...
Ch. 6 - Prob. 6.6PSCh. 6 - QUANTITATIVE More Enzyme Kinetics. The galactose...Ch. 6 - QUANTITATIVE Turnover Number. Carbonic anhydrase...Ch. 6 - Inhibitors: Wrong Again. For each of the following...Ch. 6 - What Type of Inhibition? A new mucinase enzyme was...Ch. 6 - Prob. 6.11PSCh. 6 - Prob. 6.12PSCh. 6 - Prob. 6.13PS
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Question #1: Choanoflagelletes are a unicellular ancestor to animals. One observation to support this hypothesis is the appearance of adhesion molecules that are key to the development of multiceullarity. Bulk transport Gap junctions Animals. Adhesion, cell signaling Single-celled :} Insects, mammals, and other animals with bilateral symmetry (~10,000,000) Jellyfish and their relatives (10,000) } Sponges (10,000) Choanoflagellates (150) Despite their simple unicellular lifestyle they express adhesion molecules including cadherins and lectins (King et al., 2003) but don't seem to have molecules that are typically found in the extracellular matrix such as integrins or laminins (Williams et al., 2014). Design a microscopy experiment to test the assertion that choanoflagellates have (some) adhesion molecules and those molecules play a similar role in a closely related animal like sponges.arrow_forwardHelp please. This question is specifically asking for the identification of the biomolecules that are attached to the sphingosine core, then we need to answer what bond causes those biomolecules to be connected to the sphingosine, what reaction created that bond (maybe addition or oxidation etc.), what were the starting materials and lastly what reagents or conditions are needed for the reaction to occur. Thank you!arrow_forwardMETABOLIC PATHWAYS. Carefully analyze the diagram below. Complete the diagram below by providing the name of the pathway (in oblongs beside arrows), OR the product or the precursor (rectangles). Choose your answer from the terms provided below. Write the capital letters of your answer on the space provided. The same answer can be used more than once. starch sucrose lactose 19 20 18 glucose gly cogen glycogenoly sis 17 Urea 1 cycle glucose ribos 3 16 gluconeogenesis glycolysis some amino acids 4 lactic acid amino acid catabolism 15 13 11 6 fats 12 acety FCOA ketone bodi 14 fatty add synthesis A.glucose B.fructose TERM BANK P. glycolysis Q. glycogenolysis C. galactose R. lipolys is FAD NAD+ S. ketogenesis E. pyruvate T. B-oxidation F.NADPH U. Kreb's cycle G. FADH2 V. fermentation W. glycogenesis X. lipogenesis J. glycerol Y.hydrolysis Z. oxidation AB. pentose phosphate pathway N. cellulose XY, electron transport chain D. urea 10 8 H. NADH I. NH3 9. K. H20 L. CO2 АТР M. maltose O. fatty…arrow_forward
- X Incorrect. Suppose that an uncatalyzed reaction is spontaneous because AG has a value of -10 kcal/mol. An enzyme that catalyzes the reaction is identified. What effect will the enzyme have on the rate of the reaction? Choose all that are correct. The enzyme increases the AG value. The enzyme increases the rate of reaction. The enzyme decreases the rate of reaction. The enzyme decreases the AG value. The enzyme raises the activation energy. The enzyme lowers the activation energy.arrow_forwardA. Enzyme Kinetics Two compounds were being investigated by Al Cohol, a student researcher in Biochemistry, for their inhibitory effect on xanthine oxidase (XO). The inhibition of this enzyme is critical in the treating gouty arthritis. He uses xanthine (Xan) as substrate. He investigated two inhibitors namely kaempferol (Kmp) and chlorogenic acid (Cha). The data that he was able to acquire is given in Table 1 below. Table 1. Enzyme Kinetic Data Velocity, mM/s [S], mM Хan Kmp Cha 0.492 0.0678 0.0351 0.0615 0.211 0.0531 0.0261 0.0451 0.087 0.0298 0.0157 0.0211 0.048 0.0195 0.0091 0.0142 0.029 0.0127 0.0067 0.0081 A. Construct the Lineweaver-Burke plot for each reaction system in one graph using MS Excel. Copy and paste your graph with complete regression analysis.arrow_forwardProblem. Determine KM and V, for an enzyme from the following data : max S (mM) vo (µM.s*) 1/S (mM) 1/v.(µM .s*) 2.5 1.00 0.40 2 4 0.50 0.25 5 6.3 0.20 0.16 10 7.6 0.10 0.13 20 0.05 0.11 ↑arrow_forward
- not true about the Michaelis-Menten equation? The equation that gives the rate, v, of an the substrate concentration [S] is the Michaelis-Menten equation = Vmax[S]/(Km + [S]), where V, enzyme-catalyzed reaction for all values of max and Km are constants. Which of the following is a) for [S] << Km, V = Vmax applies to most enzymes, but allosteric enzymes have different kinetics when [S] = Km, then v = Vmax/2 gives the rate when the enzyme concentration, temperature, pH, and ionic strength are constant for very high values of [S], v approaches Vmax e) Which is correct about the constant Km in the Michaelis-Menten equation? also called the catalytic constant or turnover number equal to the number of product molecules produced per unit time when the enzyme is saturated with substrate it is the constant in the first order rate equation v = k[A] it is the constant in the second order rate equation v = equal to the substrate concentration at which the velocity or rate of a reaction is ½ the…arrow_forwardI. Active site analysis. Below is a diagram of a putative active site for Monoamine oxidase. As we learned, the purpose of tertiary structure is to form a scaffold so you can orient just a few amino acids in the right orientation to promote binding and/or catalysis. The position where this occurs is the active site. The amino acid architecture of an active site is designed to bind substrates. Amino acid side chains are capable of hydrogen bonding, ionic and hydrophobic interactions. Fill in each amino acid that you think is suitable for interacting with the part of the substrate it is closest to. Assume the pH will be at 7.0 a.a.#1 a.a.#2 a.a.#6 HO Lond NH₂ НО a.a.#5 OH a.a.#3 a.a.#4arrow_forwardIn full details. Define 'activation energy' of an enzyme catalysed single substrate reaction and mention the effects of an enzyme on this energy.arrow_forward
- ENZYME KINETICS ANALYSIS of 6 Xanthine oxidase (XO) is the enzyme that catalyzes the synthesis of uric acid, which in excess causes gouty arthritis. The inhibition of this enzyme is therefore critical in its treatment. A student researcher is investigating the inhibitory effects of kaempferol (Kmp) and chlorogenic acid (Cha) on XO which uses xanthine (Xan) as substrate. Table 1 below shows the enzyme kinetic data. Construct the Lineweaver-Burk plot complete with the linear regression analvsis. Fill in the needed information on Table 2 and paste a copy of your Lineweaver-Burk plot. submit the picture of your output in PNG or JPG format. Table 1. Enzyme Kinetic Data Velocity, mM/s [S], mM Хan Kmp Cha 0.492 0.0678 0.0351 0.0615 0.211 0.0531 0.0261 0.0451 0.087 0.0298 0.0157 0.0211 0.048 0.0195 0.0091 0.0142 0.029 0.0127 0.0067 0.0081 Table 2. Enzyme Kinetic Parameters Xanthine Kaempferol Chlorogenic acid Parameters Vmax Км Type of Inhibition Mode of Binding NA NA Lineweaver-Burk Plotarrow_forwardModified TRUE or FALSE. Write the word TRUE if the statement is correct. If the statement is false, write the incorrect underlined word/s and indicate the correct word/s to make the statement true. The reaction involving the substrate bound to the active site of an enzyme is made more favorable by increasing the activation energy of the reaction.arrow_forwardBiochemical Reactions Using good details, compare and contrast the pairs of different biochemical reactions. Create your own comparing and contrasting map similar to the one below to show your understanding. Anabolism Catabolism First Pair of Describe Describe differences here différences here Reaction Types Describe similarities here Second Describe Pair of differences here Reaction Types Describe similarties here Describe differences here Pair of Reaction Describe sampar lus heru Types Describe Par of 110 Describe differences here Describe différences here Describe differences herearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education
Enzyme Kinetics; Author: MIT OpenCourseWare;https://www.youtube.com/watch?v=FXWZr3mscUo;License: Standard Youtube License