
Biochemistry: Concepts and Connections (2nd Edition)
2nd Edition
ISBN: 9780134641621
Author: Dean R. Appling, Spencer J. Anthony-Cahill, Christopher K. Mathews
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 2P
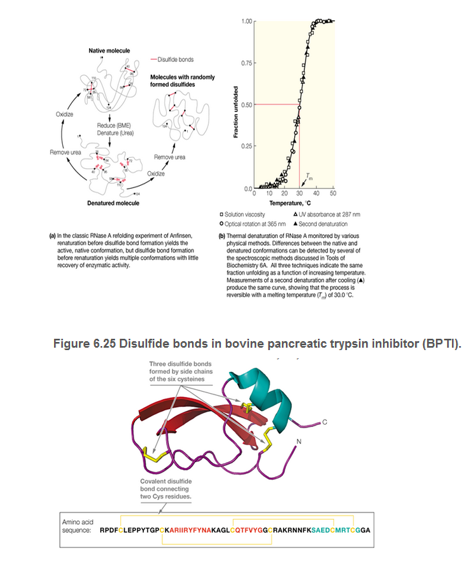
Bovine pancreatic trypsin inhibitor (BPTI; Figure 6.25) contains six cysteine residues that form three disulfide bonds in the native structure of BPTI. Suppose BPTI is reduced and unfolded urea (as illustrated for RNase A in Figure 6.22). If the reduced unfolded protein were oxidized prior to the removal of the urea, what fraction of the resulting mixture would you expect to possess native disulfide bonds?
Figure 6.22 The denaturation and refolding of ribonuclease A.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Carboxypeptidase Y (CPY) contains ten cysteine residues that form five disulfide bonds in the native structure of CPY. Suppose CPY is reduced and unfolded in urea.Part AIf the reduced unfolded protein were oxidized prior to the removal of the urea, what fraction of the resulting mixture would you expect to possess native disulfide bonds?Express your answer using three significant figures.
Globular proteins with multiple disulfide bonds must be heated longer and at higher temperature to denature them. Bovinepancreatic trypsin inhibitor (BPTI), having 58 amino acids in a single chain and 3 disulfide linkages, loses its catalytic activity whenheated at nearly 90°C for 5-10 minutes. Explain the molecular basis of this observed thermal property of BPTI relative to the nativestructure and function of the protein.
Globular proteins with multiple disulfide bonds must be heated longer and at higher temperature to denature them. Bovinepancreatic trypsin inhibitor (BPTI), having 58 amino acids in a single chain and 3 disulfide linkages, loses its catalytic activity whenheated at nearly 90°C for 5-10 minutes. Explain the molecular basis of this observed thermal property of BPTI relative to the nativestructure and function of the protein.
do not coy from other answers here
Chapter 6 Solutions
Biochemistry: Concepts and Connections (2nd Edition)
Ch. 6 - Prob. 1PCh. 6 - Bovine pancreatic trypsin inhibitor (BPTI; Figure...Ch. 6 - A schematic structure of the subunit of...Ch. 6 - In the protein adenylate kinase, the C-terminal...Ch. 6 - Give two reasons to explain why a proline residue...Ch. 6 - Consider a small protein containing 101 amino acid...Ch. 6 - a. Based on a more conservative answer to Problem...Ch. 6 - The following sequence is part of a globular...Ch. 6 - a. A protein is found to be a tetramer of...Ch. 6 - Under physiological conditions, the protein...
Ch. 6 - Theoretical and experimental measurements show...Ch. 6 - The peptide hormone vasopressin is used in the...Ch. 6 - A protein gives under conditions of buffer...Ch. 6 - A protein gives a single band on SDS get...Ch. 6 - It has been postulated that the normal...Ch. 6 - Below are shown two views of the backbone...Ch. 6 - Do you expect a Pro Gly mutation in a...Ch. 6 - Rank the following in terms of predicted rates...Ch. 6 - Shown below are two cartoon views of the small...Ch. 6 - Prob. 20PCh. 6 - In most cases, mutations in the core of protein...Ch. 6 - A Leu Ala mutation at a site buried the core of...Ch. 6 - Disulfide bonds have been shown to stabilize...Ch. 6 - Cartoon renderings of the proteins Top 7 and adaH2...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Carboxypeptidase, which sequentially removes carboxyl-terminal amino acid residues from its peptide substrates, is a single polypeptide of 307 amino acids. The two essential catalytic groups in the active site arefurnished by Arg 145 and Glu 270 .(a) If the carboxypeptidase chain were a perfect α helix, how far apart (in Å) would Arg 145 and Glu 270 be? (b) Explain how the two amino acid residues can catalyze a reaction occurring in the space of a few angstromsarrow_forwardConsider a protein with two surface-exposed histidine residues: HisA is a “typical” histidine residue with a pKa = 6.2 HisB is involved in a stabilizing interaction (His-NH+ ..... -O2C-Glu) with a neighboring glutamic acid residue. For HisB, the Gibbs free energy of deprotonation at pH = 7.0 and T = 293K is ΔG'o = +15 kj mol-1. If you had a solution, at pH = 7.0 and T = 293K, containing this protein: a) What fraction of HisA residues are protonated? b) What fraction of HisB residues are protonated? c) What is the pKa of HisB?arrow_forwardWhen performing his experiments on protein refolding, Christian Anfinsen obtained a quite different result when reduced ribonuclease was reoxidized while it was still in 8 M urea and the preparation was then dialyzed to remove the urea. Ribonuclease reoxidized in this way had only 1% of the enzymatic activity of the native protein. Why were the outcomes so different when reduced ribonuclease was reoxidized in the presence and absence of urea?arrow_forward
- Some of the following four amino acids : alanine, arginine, histidine, aspartic acid would provide a side chain for acid-base catalysis at physiological pH (assume pK of each amino acid is equal to pK value for the free amino acid in solution). Explain for each amino acid how and why each would or would not provide the side chain residue to support acid-base catalysis at physiological pH.arrow_forwardTryptophan is not classified as a basic amino acid even though it has aheterocycle containing a nitrogen atom. Why is the N atom in the fivemembered ring of tryptophan not readily protonated by acid?arrow_forwardBased on your knowledge of the natural amino acids, what would you expect pKa of the sidechain of beta-(aminomethyl)succinic acid to be? Draw an appropriate titration curve for beta-(aminomethyl)succinic acid, labeling the axes, indicating the equivalence points and the pKavalues, and use a drawing to show the protonated/deprotonated forms.arrow_forward
- A newly discovered blob protein folds very rapidly in the presence of protein disulphide isomerase and peptidyl prolyl cis-trans isomerase enzymes but aggregates and never folds correctly without protein disulphide isomerase. Explain why this might occur.arrow_forwardExperimental results describing a protein's amino acid composition are useful for estimating the molecular weight (MW) of the entire protein. A quantitative amino acid analysis reveals that bovine cytochrome c contains 1% tryptophan (M, 204) by weight. Calculate the approximate molecular weight of bovine cytochrome c if there is 1 tryptophan residue. Please enter your answer with three significant figures. approximate bovine cytochrome c MW: number of threonine residues: 19.4 20.4 x10³ Bovine chymotrypsinogen has a molecular weight of 25.6 kDa. Amino acid analysis shows that this enzyme is 9% threonine (M, 119). Calculate how many threonine residues are present in a molecule of bovine chymotrypsinogen. Round your answer to the nearest whole number. Incorrect Incorrect Daarrow_forwardConsider the phenolic hydroxyl group of a particular Tyr residue in a protein. Suppose the hydroxyl group in the unfolded protein in aqueous solution, where the group is exposed to H2O, has a pKa of 10.0. If that group is found in a hydrophobic environment in the interior of the protein when the protein is folded into its native tertiary structure, would you expect the pKa of the phenolic hydroxyl to be higher or lower in the folded protein interior than in H2O? Explain your reasoning.arrow_forward
- What is the smallest number of molecules of ATP and GTP consumed in the synthesis of a 200- residue protein, starting from amino acids? Assume that the hydrolysis of PP i is equivalent to the hydrolysis of ATP for this calculation.arrow_forwardEukaryotic protein X has one proline residue and two cysteine residues. The proline needs to be in the cis-configuration in the folding protein; and a disulphide bond is formed between the cysteines in the folded protein. Discuss the folding of the protein in solution with protein disulphide isomerase and peptidyl-prolyl isomerase and without these enzymes. Use illustrations to show the differences in the potential folding of protein X under the different conditions.arrow_forwardWhen protein cannot precipitate out under the conditions for making tofu and cheese because (i) polar groups are highly abundant in the surfaces of whey proteins; and (ii) disulfide bonds are commonly present in the structures of whey proteins. Explain briefly why highly abundant polar groups and disulfide bonds could make whey proteins resist precipitation of protein molecules. (i) Under the condition where the environmental pH is equal to the isoelectric point of whey protein (ii) Under the condition where rennet is added to whey protein solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON
Macromolecules | Classes and Functions; Author: 2 Minute Classroom;https://www.youtube.com/watch?v=V5hhrDFo8Vk;License: Standard youtube license