
Concept explainers
Under physiological conditions, the protein hemerythrin exists as an octamer of eight chains of the kind shown in Problem 3.
a. Name two symmetries possible for this molecule
b. Which do you think is more likely? Explain.
c. For the more likely symmetry, what kinds of interactions (isologous, heterologous, or both) would you expect? Why?
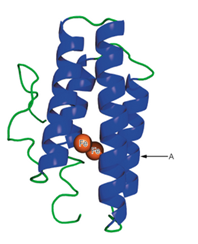
3. A schematic structure of the subunit of hemerythrin (an oxygen-binding protein from invertebrate animals) is shown to the right.
a. It has been found that in some of the a-helical regions of hemerythrin, about every third or fourth amino acid residue is a hydrophobic one. Suggest a structural reason for this finding.
b. What would be the effect of a mutation that placed a proline residue at point A in the structure?
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Biochemistry: Concepts and Connections (2nd Edition)
- 16.2arrow_forward3) You are working on a protein with the following sequence in an area of interest. -Asp-Leu-Leu-Gln-Glu-Glu-Asp-Glu-Ser-Arg a. The current structure, solved at pH 7.4, of this segment has an alpha helix that is disrupted a er the Gln. Why might the alpha helix stop at this residue? b. This protein is involved in Lysosomes in vivo. The secondary structure of this region is expected to change into a complete alpha helix. Why might this change into a complete helix? ( Hint: Lysosomes are acidic!)arrow_forwardConsider an a-helix secondary structure.a. Propose a 12-residue amino acid sequence that(assuming it folded intoan a-helix) would yield a structure in which six of the residues, each with an ionizable side-chain were within a 135° region on one face of the helix, and use an appropriate diagram to support your choice of sequence.b. Would it be possible to generate an especially stable helix by correctly choosing the charges on the side-chains? Please justify your answer.arrow_forward
- Certain polypeptide sequences that show a pronounced tendency to dimerize in an antiparallel coiled-coil structure exhibit an exact repetition of leucine every 7 residues. If you also know that the a helix is distorted somewhat from its usual 3.6 residues/turn in coiled coils, propose a mechanism for the dimerization. What does this suggest that the residue/turn value might be in a coiled coil?arrow_forwardAt physiological pH (i.e. 7.4), polylysine assumes a random structure in solution. Given that the pKa for the e-amino group of lysine is 10.5, under what conditions would you expect polylysine to form an a-helix? Explain your reasoning. Under what conditions would you expect polyglutamate to form an a-helix, given that the pKa for the side-chain COOH group is 4.3?arrow_forward- (a) A protein is found to be a tetramer of identical subunits. Name two symmetries possible for such a molecule. What kinds of interac- tions (isologous or heterologous) would stabilize each? (b) Suppose a tetramer, like hemoglobin, consists of two each of two types of chains, a and B. What is the highest symmetry now possible?arrow_forward
- 2. 3 With the help of the internet and your textbook, A. hand-draw the chemical structures of dipalmitoyl phosphatidyl choline, DPPC (also known as lecithin, although lecithin is a more general name and can refer to other chemical structures). B. Where is DPPC found in a cell? 4 C. Is this a lipid, carbohydrate, protein or nucleic acid? Do not leave any "R" groups in the structure that you draw. With the help of the internet and your textbook, hand-draw the chemical structures of the following: A. ibuprofen (What trademarked product is this used in?) B. amoxicillin (What type of agent is this?) C. diltiazem (What condition is this used to treat?) D. L-aspartyl-L-phenylalanine methyl ester (What is this chemical's trade name?) E. atorvastatin (What trademarked product is this used in?) Draw each of these structures by hand. Do not leave any "R" groups in the structure that you draw. Using the structures you drew in questions 2 & 3, for each of the organic functional groups listed below,…arrow_forwardUsing the quarternary structure of hemoglobin shown inFigure 9-3(d), explain in structural terms how a mutation in the β subunit protein could be suppressed by amutation in the a subunit gene.arrow_forwardWhen protein X is subjected to dimethylsuberimidate and SDS electrophoresis a 50 kDa band is seen; when the protein X is subjected to SDS electrophoresis without DMS, two bands of 20 kDa and 10 kDa are seen. How many different oligomeric structures of the native protein X are possible, being consistent with these observed results? one two three none of the above which is the answrr? and explainarrow_forward
- The dissociation constant, Kd for a complex between protein A and protein B is 4.1 μM. If the two proteins are mixed together at initial concentrations of [A]= 0.025 μM and [B] = 4.7 μM, calculate (a) the equilibrium concentrations of A, B, and AB (the dimer formed by A and B) (b) the percentage of A bound to Barrow_forwardA synthetic polypeptid made up of L-glutamic acid residues is in a random coil configuration at pH 7.0 but changes to alpha helical when the pH is lowered to 2.0. Explain this pH-dependent conformational transition.arrow_forward2arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





