
Biochemistry: Concepts and Connections (2nd Edition)
2nd Edition
ISBN: 9780134641621
Author: Dean R. Appling, Spencer J. Anthony-Cahill, Christopher K. Mathews
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 6, Problem 12P
The peptide hormone vasopressin is used in the regulation of saltwater balance in many vertebrates. Porcine (pig) vasopressin has the sequence
Asp-Tyr-Phe-Glu-Asn-Cys-Pro-Lys-Gly
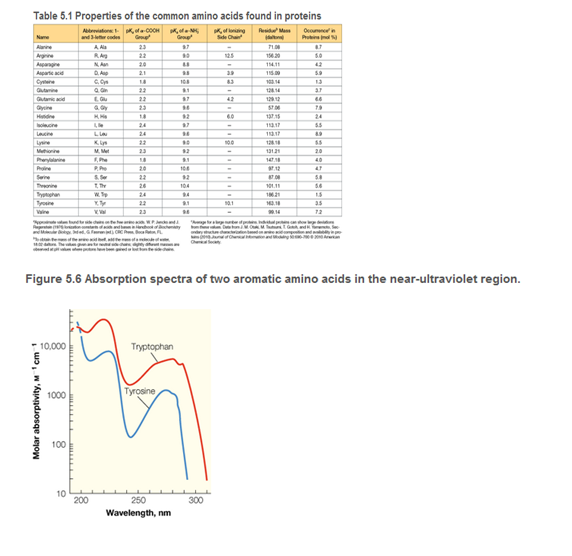
a. Using the data in Figure 5.6 and Table 5.1, estimate the extinction coefficient e (in units of cm2/mg) for vasopressin, using radiation with λ = 280 nm.
b A solution of vasopressin is placed in a 0.5-cm-thick cuvette. Its absorbance at 280 nm is found to be 1.3. What is the concentration of vasopressin, in mg/cm3? (See Tools of Biochemistry 6A).
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Penicillin is hydrolyzed and thereby rendered inactive by penicillinase, an enzyme present in some resistant bacteria. The mass of this enzyme in Staphylococcus aureus is 29.6 kd. The amount of penicillin hydrolyzed in 1 minute in 10 ml solution containing 10–9 g of purified penicillinase was measured as a function of the concentration of penicillin. Assume that the concentration of penicillin does not change during the assay.
[Penicillin] µM Amount hydrolyzed (nanomoles)
1 0.11
3 0.25
5 0.34
10 0.45
30 0.58
50 0.61
a) Plot V0 versus [S] and 1/V0 1/[S]. Does penicillinase appear to obey Michaelis-Menten kinetics?
b) What is the value of KM?
c) What is the value of Vmax?
d) What is the turnover number of penicillinase under these conditions?
80mL of a 0.3M solution of hexapeptide Leu-His-Cys-Glu-Asn-Arg is adjusted
to pH=pl. The solution is then titrated with 0.2M HCI to a final pH of 2.1.
Sketch the titration curve, labelling the pH and volume axes. Indicate the
volume of HCl needed to reach each relevant pKa value and equivalence
point(s). Relevant pka values are: 2.1, 4.3, 6.0, 8.3, 9.8, and 12.5.
Polymer beads (resin) made of DEAE (diethylaminoethyl) cellulose are packed in an ion exchange column. The
total mass of beads in the column is 8.47 kg. On average, each bead weighs 0.0023 g and has an average of 18.4
* 10° positively charged amine groups that can adsorba negatively charged protein that passes through the
column. A solution containing 2.07 mg/L of a protein is maintained at pH 6.3 and is passed through the ion
exchange column at 0.215 L/min. The protein has a molecular weight of 154,000. The pk, of the amino groups
on DEAE cellulose is 7.1, and the pl of the protein is 5.6.
2.
A. How long can the column be operated before reaching 80% capacity (i.e., 80% of the amino groups on DEAE
are bound to the protein through an ionic bond)? You may assume that one protein attaches to one +
charge on the beads (although it's possible that proteins attach to more than one + charge).
B. After reaching 80% capacity, explain what you would do to release the protein attached to the…
Chapter 6 Solutions
Biochemistry: Concepts and Connections (2nd Edition)
Ch. 6 - Prob. 1PCh. 6 - Bovine pancreatic trypsin inhibitor (BPTI; Figure...Ch. 6 - A schematic structure of the subunit of...Ch. 6 - In the protein adenylate kinase, the C-terminal...Ch. 6 - Give two reasons to explain why a proline residue...Ch. 6 - Consider a small protein containing 101 amino acid...Ch. 6 - a. Based on a more conservative answer to Problem...Ch. 6 - The following sequence is part of a globular...Ch. 6 - a. A protein is found to be a tetramer of...Ch. 6 - Under physiological conditions, the protein...
Ch. 6 - Theoretical and experimental measurements show...Ch. 6 - The peptide hormone vasopressin is used in the...Ch. 6 - A protein gives under conditions of buffer...Ch. 6 - A protein gives a single band on SDS get...Ch. 6 - It has been postulated that the normal...Ch. 6 - Below are shown two views of the backbone...Ch. 6 - Do you expect a Pro Gly mutation in a...Ch. 6 - Rank the following in terms of predicted rates...Ch. 6 - Shown below are two cartoon views of the small...Ch. 6 - Prob. 20PCh. 6 - In most cases, mutations in the core of protein...Ch. 6 - A Leu Ala mutation at a site buried the core of...Ch. 6 - Disulfide bonds have been shown to stabilize...Ch. 6 - Cartoon renderings of the proteins Top 7 and adaH2...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider a uniport system where a carrier protein transports an uncharged substance A across a cell membrane. Suppose that at a certain ratio of [A]inside to [A]outside, the AG for the transport of substance A from outside the cell to the inside, Aoutside → Ainside, is -11.3 kJ/mol at 25°C. What is the ratio of the concentration of substance A inside the cell to the concentration outside? [A]inside [A]outside = Choose the true statement about the transport of A under the conditions described. Increasing [A]outside will cause AG for movement of Aoutside to Ainside to become a smaller negative number. Decreasing the concentration of the uniport protein in the membrane will cause AG to become a larger negative number. Movement of Aoutside to Ainside will be spontaneous. Because AG is negative, the ratio [A]inside/[A]outside must be greater than one.arrow_forwardConsider a uniport system where a carrier protein transports an uncharged substance A across a cell membrane. Suppose that at a certain ratio of [A]inside to [A]outside, the AG for the transport of substance A from outside the cell to the inside, Aoutside → Ainside, is -12.1 kJ/mol at 25°C. What is the ratio of the concentration of substance A inside the cell to the concentration outside? [A]inside [A]outside || 656275.63 Incorrect Choose the true statement about the transport of A under the conditions described. Increasing [A]outside will cause AG for movement of Aoutside to Ainside to become a smaller negative number. Decreasing the concentration of the uniport protein in the membrane will cause AG to become a larger negative number.arrow_forwardPhosphoproteins are formed when a phosphate group is esterified to an -OH group of a Ser, Thr, or Tyr side chain. At typical cellular pH values, this phosphate group bears two negative charges (-OPO32-). Compare this side-chain modification to the 20 side chains of the common amino acids found in proteins and comment on the novel properties that it introduces into side-chain possibilities.arrow_forward
- Diethylaminoethyl cellulose is a positively charged resin used in ion-exchange chromatography with a pKa is about 11. A negatively charged protein with an isoelectric point of 5.0 is applied to the column in a buffer at pH 7 and containing 0.1 M NaCl. Which of the following conditions is most likely to weaken the interaction between the protein and the resin? A Raising the pH to 8 and decreasing the NaCl to 0.05 M B Raising the pH to 8 and increasing the NaCl to 0.2 M C Lowering the pH to 6 and decreasing the NaCl to 0.05 M D Lowering the pH to 6 and increasing the NaCl to 0.2 Marrow_forwardThe hexapeptide Ala-Met-Leu-Lys-Phe-Asp is digested in the same tube with both Cyanogen Bromide and Trypsin at the same time at pH=7. Draw the structure of the product(s) that would bind to a cation exchange column. (Relevant pKa values are 2.2, 3.9, 9.5 and 10.5. Assume pKa values for any newly generated a-amino and a-carboxyl groups are 9.5 and 2.2 respectively)arrow_forwardYou are given a pure protein sample to characterize and provided the following information: Its molar extinction coefficient, ε280, is 0.25 liters micromole-1 cm-1 in both the folded and unfolded form Its ΔGo for unfolding is 1.5 kcal/mol at 37o (where RT = 0.59 kcal/mole) A) Using a 0.5 cm pathlength cell, you measure the absorbance at 280 nm of a 20-fold dilution of your pure protein in solution (by this, we mean that 50 ul of the protein sample was diluted to a final volume of 1 ml) and find A280 = 0.40. What is the original concentration of the protein before dilution? B) What is the concentration of the unfolded form of the protein in your sample?arrow_forward
- Human blood serum contains a class of enzymes known as acid phosphatases, which hydrolyze biological phosphate esters under slightly acidic conditions (pH 5.0): R-O-P-O3-2 + H2O --> R-OH + HO-P-O3-2. Acid phosphatases are produced by erythrocytes, the liver, kidney, spleen, and prostate gland. The enzyme from the prostate gland is clinically important because an increased activity in the blood is frequently an indication of cancer of the prostate gland. The phosphatase from the prostate gland is strongly inhibited by tartrate ion, but acid phosphatases from other tissues are not. How can this information be used to develop a commercial specific procedure for measuring the activity of the acid phosphatase of the prostate gland in human blood serum? * 1. Prostate cancer cannot be diagnosed biochemically. 2. Use tartrate to inhibit phosphatase from prostate gland and then subtract the results from the total serum enzyme activities to get an…arrow_forwardA set of biomolecules listed in the table at right are in solution at pH 6.8, when they are passed through the multi-step separation process shown below. The sample passes through an ultrafilter with MWCO of 50,000 (assume this filter gives a perfect separation of molecules above and below the MWCO), and the retentate then flows through an ion exchange column filled with beads that have a positive charge under the operating conditions (pks = 7.3). Inlet Biomolecule Mixture pH 6.8 Ultrafiltration MWCO 50,000 Permeate Retentate lon Exchange Column Biomolecule Molecular weight pl A 68,000 4.2 B 92,600 9.6 C 144,000 A-3. Which biomolecules (A, B, C, etc.) exit the process in the permeate stream? K C-3. Which biomolecules are retained inside the ion exchange column? D E F G H B-3. Which biomolecules exit the process after passing through the ion exchange column? 5,800 68,000 Packed with DEAE cellulose beads, which have positively charged groups below a pH of 7.3 156,000 45,000 16,000 None…arrow_forwardThe diffusivity of amino acids in polyacrylamide gel is approximately 1x10^-9 cm2./s calculate the initial flux of amino acids, give an instantaneous gradient of (20g/cm 3 )/8cm Why is polyacrylamide gel is used in electrophoresis?arrow_forward
- Calculate the solubility of Ag,CO3 (in mol/L) at 25 °C in a 0.02 M Na2CO3 solution. Hint: Ag,CO3(s) → 2Ag*(aq) + co3?(ag) Ksp (Ag,CO3) = 8.1 x 10 12 NażCO3(aq) → 2Na*(aq) + CO3 (aq)arrow_forwardThe following table shows the molecular masses (Mr) and isoelectric points (pI) of five proteins: Protein Mr pI Chicken egg white lysozyme 14,000 11.0 Carbonic anhydrase 30,000 5.4 Ovalbumin 45,000 4.5 Phosphorylase b 97,000 6.7 Lactate dehydrogenase 140,000 7.12 (a) A solution containing these five proteins was adjusted to pH 7.0 and then applied to a SIZE-EXCLUSION COLUMN. Assume that these five protein molecules are spherical in shape. Which protein is likely to be eluted LAST from the column? Explain briefly. (b) In another experiment, this protein mixture was first adjusted to pH 6.7 before applying to a CATION-EXCHANGE COLUMN. (i)What is the net charge on phosphorylase b? (ii) Which of these five proteins will bind to the CATION-EXCHANGER? Explain briefly.varrow_forwardA tetrapeptide, glutamate-glycine-alanine-lysine, is prepared at at concentration of 1 mM (0.001 M) and is measured in the standard setup (pathlength of 1 cm). What is the approximate absorbance of this peptide at 280 nm? Hint: if the peptide contained a single tryptophan, the answer would be about 10. 10 280 1 0arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON
GCSE Chemistry - Acids and Bases #34; Author: Cognito;https://www.youtube.com/watch?v=vt8fB3MFzLk;License: Standard youtube license