
Concept explainers
(a)
Interpretation: Whether the proposed statement by the student about the bond shown below as triple bond should be explained with appropriate argument.
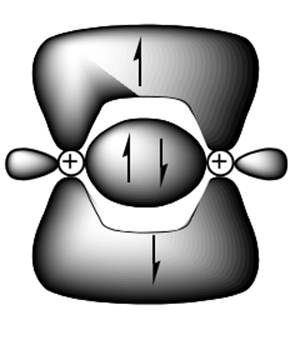
Concept introduction: Two or more of orbitals undergo redistributions of their different energies so as to form mathematically averaged orbitals in terms of energy. This phenomenon is referred as hybridization.
A single bond has one
(b)
Interpretation: The number of electrons present in a triple bond and in one representation shown below should be indicated.
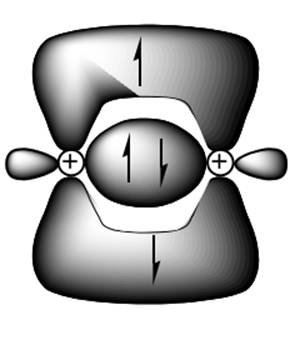
Concept introduction: Two or more of orbitals undergo redistributions of their different energies so as to form mathematically averaged orbitals in terms of energy. This phenomenon is referred as hybridization.
A single bond has one
(c)
Interpretation: Misconception that might have led to bond identified as triple bond by the student should be known.
Concept introduction: The 2 types of overlapping are as follows:
1. Head-on overlapping (sigma bond)
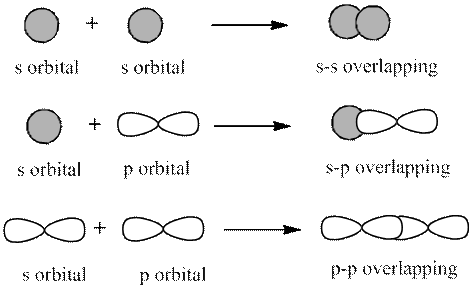
2. Sideway overlapping (pi bond)
A single bond has one
(d)
Interpretation: The space that could be used to add another p bond so as to generate a triple bond should be determined.
Concept introduction: Two or more of orbitals undergo redistributions of their different energies so as to form mathematically averaged orbitals in terms of energy although they may differ in shape and orientation. This phenomenon is referred as hybridization.
The energy and orientation of the new hybrid orbital depend upon by the kind and number of orbitals used in the hybridization. The new hybrid orbitals are always equal in number to number of atomic orbitals that combine.
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Organic Chemistry: A Guided Inquiry
- How many valence electrons does a neutral a. K atom have? b. C atom? N atom? O atom?arrow_forwardChoose the best ending to this statement, given what you have learned about MO Theory. "When two orbitals combine to make a bond... O ..two new orbitals are formed. One is lower energy than the atomic orbitals and the other is higher energy than the starting atomic orbitals." O .only one new orbital is formed which holds the bonding electrons." O .two new orbitals are formed. Both new orbitals are lower energy than the starting orbitals. O .the number of orbitals formed depends on how many electrons are shared between the two atoms."arrow_forwardHelp me pleasearrow_forward
- Use the following scheme to answer the next three problems. OH HgCl₂ H₂O C 1. BuLi 2. compound D. 3. H HS BF3 SH Earrow_forwardMolecular geometry. Don’t know what I’m doing wrong here.arrow_forwardFill in the blank. Adding electrons into an antibonding orbital the bond strength. A. decreases B. increases C. has no effect on D. changesarrow_forward
- What is delocalization energy? How is it related to resonance energy? Answer by selecting all true statements. 00 Resonance energy is a term used in VB theory. Resonance energy and delocalization energy are term that describe different conditions. Resonance energy is a term used in MO theory. Resonance energy and delocalization energy are essentially the same thing. Resonance energy and delocalization energy represent the additional stability associated with a spreading out of electron density. Delocalization energy is a term used in VB theory. Delocalization energy is a term used in MO theory. Resonance energy and delocalization energy represent the additional stability associated with concentrating electron density.arrow_forwarda. Label the hybridization of the identified atoms (A.-F.). [Note: All bonds have been identified for these letters.] TT b. Write the total number of o and n bonds in the structure. [Note: All bonds have NOT been identified for all atoms in the molecule. You need to fill in the bonds recognizing that at the end of every line, unless already designated with another atom, and every juncture between two lines is a carbon atom. And carbon makes four bonds around it at all times, so if a bond is not shown, it still exists and is to an H atom.] OH O A. H HOE CH 3 B. C ): CH 3 D. H F. 10: 5: E. Harrow_forward7. Predict the polarity of 6 real molecules. First, draw the molecules and any bond dipoles. Then draw any molecular dipoles. Explain your reasoning before you check your predictions with the simulation.arrow_forward
- Which statement A-D about VSEPR theory is not correct? Select one: a. The steric number has five values from 2 to 6. b. Statements A-D are all correct. c. In VSEPR theory, the shape or geometry of a molecule is determined by electron-electron repulsion. d. The steric number of a central atom is the sum of the number of bonds and lone pairs around the atom. e. The molecular shape or geometry can differ from the electron-pair geometry.arrow_forwardH. H H. H. molecule> H. atoms. Select only one type of bond in each molecule. If there are multiples of one bond type for a molecule, select them all. Do not highlightarrow_forwardExamine the picture in the box below. Z Select the atomic orbitals in the table below that would blend together to produce the set of hybrid orbitals pictured in the box. If at least one orbital is missing to make the pictured hybrid set, select "missing atomic orbitals". Z -X -X -x Z missing atomic orbitals X Ś 00. Ararrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

