
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 5E
Consider the following orbital representation of HCCH (ethyne).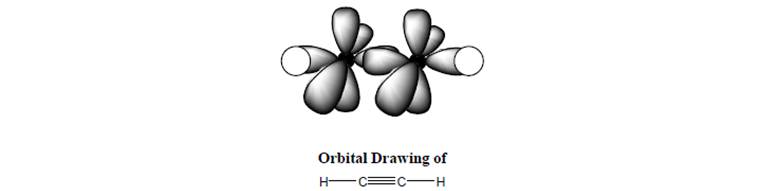
a. Answer the same three questions (a-c) from the previous exercise.
b. Label each
c. What is the total number of a bonds found in ethyne?….
d. How many p orbitals are there on a single carbon of ethyne?
e. How many hybrid orbitals are there on a single carbon of ethyne?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1. Is the following orbital sigma or pi? Bonding or antibonding? (circle your answers). The orbital is
between two carbons, the nuclei of which are roughly where the black circles are.
2. What atomic orbitals would be used to make the orbital from question 2?
3. Label the hybridization of each indicated atom in the following molecule as SP, SP³, or SP³,
OH
4. Circle the shortest carbon-carbon single bond in the above molecule (Advanced question).
Explain why it is the shortest in a sentence or two.
5. Draw the major resonance structures of the following molecule
S
H₂N
Aromatic hydrocarbons
-9. C6H6 (benzene)
Projection Drawing:
Perspective Drawing:
Draw the two resonance forms
a. Predict the angle CCC.
b. predict the angle H-C-C
10. methylbenzene (toluene)
Projection Drawing:
Perspective Drawing:
Draw the two resonance forms
a. Predict the angle CCC.
b. predict the angle H-C-C
B. Hydrocarbon Derivatives
ucture
11. Chloropropane
Projection Drawing:
Perspective Drawing:
Draw the two resonance forms
a. Predict the angle CCC.
b. Predict the angle Cl-C-C.
17
2. Provide the following answer using the following compound below.
a. Assign the hybridization for each atom, except hydrogen.
b. Determine the number of atomic orbitals and Molecular orbitals (AO, MO).
c. Draw the atomic orbitals for each atom and their MO
d. Draw the energy diagram.
e. Assign the HOMO and LUMO in the energy diagram.
H
H-C-C=C-H
FO:
H
Chapter 3 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 3 - Prob. 1CTQCh. 3 - What neutral atom is represented by the electron...Ch. 3 - Prob. 3CTQCh. 3 - Consider any one of the four identical hybrid...Ch. 3 - Prob. 5CTQCh. 3 - Prob. 6CTQCh. 3 - Prob. 7CTQCh. 3 - Prob. 8CTQCh. 3 - Prob. 9CTQCh. 3 - Prob. 10CTQ
Ch. 3 - On the left side of Figure 3.6, label the areas...Ch. 3 - Prob. 12CTQCh. 3 - Prob. 13CTQCh. 3 - Prob. 14CTQCh. 3 - Prob. 15CTQCh. 3 - Now consider the fully formed molecule on the...Ch. 3 - Prob. 1ECh. 3 - Explain why the two molecules below cannot...Ch. 3 - Prob. 3ECh. 3 - Consider the incomplete orbital representation of...Ch. 3 - Consider the following orbital representation of...Ch. 3 - Summarize how one determines the hybridization...Ch. 3 - Explain what is wrong with each of the following...Ch. 3 - Prob. 8ECh. 3 - Prob. 9ECh. 3 - Complete the following tables, and memorize their...Ch. 3 - Draw orbital representations of bonding in water...Ch. 3 - Draw electron configuration diagrams for carbon in...Ch. 3 - Prob. 13E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Now consider the fully formed molecule on the right side of Figure 3.7. a. Draw a Lewis structure of this molecule. b. Identify orbital representations of the two bonds and three bonds in Figure 3.7, andmatch these with representations of bonds on the Lewis structure you just drew.arrow_forward2. Provide the following answer using the following compound below. a. Assign the hybridization for each atom, except hydrogen. b. Determine the number of atomic orbitals and Molecular orbitals (AO, MO). c. Draw the atomic orbitals for each atom and their MO d. Draw the energy diagram. e. Assign the HOMO and LUMO in the energy diagram. H H-C-C=C-H :O: Harrow_forward1) For each condensed or skeletal structure below: A. Draw a complete Lewis structure. Note: show all non-bonding electron pairs. B. Indicate the hybridization of all C, O, and N atoms in each of the molecules. C. Label each of the indicated bonds (marked by an arrow) as either a sigma or pi bond. (Note: Remember, e.g. that a double bond is made up of both a o and a π bond). Also indicate which orbitals overlap to form the bond (e.g., Ols-2sp3). D. For atoms having lone pairs in the molecules below, indicate in which orbitals the lone pairs are located. E. Which of the bonds indicated by arrows is shorter? a) c) H CH3 C=CHC=C-CH g) (CH3)3CCN CH3 CH₂CH=CHCECH b) d) CH3 Lo LOH f) CH₂CH(CH3)SH h) (CH3)2CH(CH₂)3Brarrow_forward
- Tag all the sp hybridized carbon atoms in this molecule. If there are none, please check the box below. H. H. H C C-CEN: H. H. O There are none.arrow_forwardAnswer the following questions about amoxicillin, an antibiotic from the penicillin family. NH2 H N. HO НО amoxicillin a. Predict the hybridization and geometry around each highlighted atom. b. Label five polar bonds using the symbols &+ and &–. C. How many r bonds does amoxicillin have? Label them. d. Find a C – H bond containing a carbon atom having a hybrid orbital with 33% s-character.arrow_forwardThis question asks you to describe sigma or pi bonds as being derived from the overlap of hybridized orbitals. Describe each bond indicated as the overlap of hybridized orbitals. For example, for a C-O bond, an answer might be a Csp3-Osp3 signma bond and you can answer like: #A: Csp3-Csp3 sigma bond Enter you answers in the box below CH3 H, .C A H.arrow_forward
- I NEED ANSWER FOR NUMBER 3. Find the following in each compound :A. Draw the overlapping of orbitalsB. Identify the hybrid orbitalC. Identify the molecular orbitalarrow_forwardEvery atomic orbital, every molecular orbital has a particular name, such as s orbital, p orbital, d orbital, s orbital, p orbital, s* orbital, p* orbital, etc. a. What are the names of the atomic orbitals in 5d above? b.What is the name of the molecular orbital in 5f abovearrow_forward11. What is a phosphorous ylide? A. a negatively charged carbon attached to a positively charged phosphorous B. a negatively charged phosphorous attached to a positively charged carbon C. an sp hybridized carbon attached to a phosphorous D. a phosphorous attached to a double bonded carbon on each sidearrow_forward
- 1. Do all of the following for each molecule or ion. a. Draw the Lewis Dot structure that minimizes formal charges. b. Draw the 3-D structure. c. Determine the Hybridization designation of the central atom. d. Determine the VSEPR designation. e. State what is its Molecular shape. f. Determine whether it is Polar (P) or Nonpolar (N). g. Determine the number of sigma (0) and pi (0) bonds in each structure А. РОд 3- B. SeF4 C. CH,O D. BrF5 Е. СН2arrow_forward1. For each set of molecules below circle the sets that represent valid resonance forms. Be sure to show the correct electron flow arrows to support your answer. HN. HN 2. Consider the following molecule. Answer the questions pertaining to this molecules structure. How many primary (1º) carbons are present in the molecule? How many tertiary hydrogens are present in the molecule? How many sp hybridized atoms are present in the molecule? How many sp² hybridized atoms are present in the molecule? How many sp' hybridized atoms are present in the molecule?arrow_forwardLet us construct the molecular orbital diagram of ethylene (in pieces). a. First, construct the MO diagram of linear carbene (CH2). Draw pictures of all 6 orbitals b. Now bend the carbene to a bond angle of about 120°. How does this change your MO diagram? Draw pictures of all 6 orbitals. c. Now bring two of these carbene molecules together to make ethylene. Draw pictures of all 12 orbitals.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Types of bonds; Author: Edspira;https://www.youtube.com/watch?v=Jj0V01Arebk;License: Standard YouTube License, CC-BY