
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 3, Problem 10CTQ
Interpretation Introduction
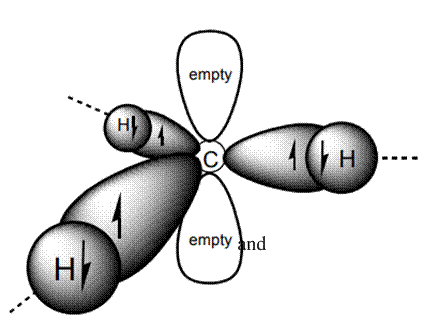
Interpretation: Total valence electrons found in cation
Concept introduction: A carbocation is a planar system and therefore
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
2.
3-42. The gas boron trifluoride (BF3) has a
trigonal planar configuration as shown
in Fig. P3.42. The B-F bond length is 1.3
Angstrom. Adjacent fluoride molecules
form a 120° angle. Find the distance
between adjacent fluoride molecules.
F
F
B
120°
F
Figure P3.42 Planar configuration of boron
trifluoride.
What is the difference between valence and core electrons, and how do they relate to atomic properties such as bonding and reactivity? (keywords: shielding) Explain in DETAIL (3-4 sentences).
Give typed full explanation
Look at figure 3-22 that shows the electron density that occurs abound the Si-O bond. This electron density map gives the "shape" of the O and Si atoms when they are bonded together. Think about the answer in Q9 and choose the best response below:
(Select answer choice)
a. This figure shows that the Si and O atoms, when they bond together, do not form spheres, which is due to the fact that the Si-O bond is strongly covalent and these shared electrons affect atomic shape. This change in shape limits the applicability of Pauling's Coordination principle since that principle is based on the geometry of perfect spheres.
b. This figure shows that the Si and O atoms, when they bond together are close to perfect spheres, which is due to the fact that the Si-O bond is strongly covalent. This figure shows that Pauling's Coordination principle should apply very precisely to any substance that contains Si-O bonds
c. This figure shows that the Si and O atoms, form in a…
Chapter 3 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 3 - Prob. 1CTQCh. 3 - What neutral atom is represented by the electron...Ch. 3 - Prob. 3CTQCh. 3 - Consider any one of the four identical hybrid...Ch. 3 - Prob. 5CTQCh. 3 - Prob. 6CTQCh. 3 - Prob. 7CTQCh. 3 - Prob. 8CTQCh. 3 - Prob. 9CTQCh. 3 - Prob. 10CTQ
Ch. 3 - On the left side of Figure 3.6, label the areas...Ch. 3 - Prob. 12CTQCh. 3 - Prob. 13CTQCh. 3 - Prob. 14CTQCh. 3 - Prob. 15CTQCh. 3 - Now consider the fully formed molecule on the...Ch. 3 - Prob. 1ECh. 3 - Explain why the two molecules below cannot...Ch. 3 - Prob. 3ECh. 3 - Consider the incomplete orbital representation of...Ch. 3 - Consider the following orbital representation of...Ch. 3 - Summarize how one determines the hybridization...Ch. 3 - Explain what is wrong with each of the following...Ch. 3 - Prob. 8ECh. 3 - Prob. 9ECh. 3 - Complete the following tables, and memorize their...Ch. 3 - Draw orbital representations of bonding in water...Ch. 3 - Draw electron configuration diagrams for carbon in...Ch. 3 - Prob. 13E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Now consider the fully formed molecule on the right side of Figure 3.7. a. Draw a Lewis structure of this molecule. b. Identify orbital representations of the two bonds and three bonds in Figure 3.7, andmatch these with representations of bonds on the Lewis structure you just drew.arrow_forwardA student draws the picture of ammonia (NH3) in the box below, left, and predicts it will be a flatmolecule with HNH bond angles of exactly 120°. Unfortunately, the student left something out. a. What did the student omit from his drawing? b. What is the actual HNH bond angle of ammonia (based on the draw g above, right)? c. Explain why water, ammonia, and methane (shown below) all have about the same bondangles (close to 109.5°) even though they have different numbers of bonds.arrow_forwardDraw a model, like those in Figure , for CH4. Hint: Carbon has two electrons in its inner shell and four in its second shell.arrow_forward
- Select all the atoms that have at least one lone (unshared) pair of electrons. • Gray = C; white H; red= 0; blue = N; dark green= Cl; brown Br; light green F; purple = 1; yellow=S; orange - P Double click to select atoms. You can zoom in and out using the mouse scroll wheel (or pinch to zoom on touch screens). Convert the model below to a skeletal drawing. wireframe H H H C H H + labels Harrow_forwardDraw the shape of the PFs molecule and answer the following questions: (i) What is the principal rotation axis of the PFs molecule? (ii) Does the molecule have other rotation axes? If so, what are the other rotation axes of the molecule? (iii) Does the molecule have improper rotation axes? If so, what is the improper rotation axis of the molecule? (iv) Does the molecule have ơ, mirror plane(s)? If so, how many? (v) Does the molecule have ơy mirror plane(s)? If so, how many? (vi) Does the molecule have ơa mirror plane (s)? If so, how many? (vii) What is the point group of the PF, molecule? (viii) What is the order of the point group of the PFs molecule? (ix) Using symmetry criteria, predict if the PFs molecule is polar or non-polar. (x) Using symmetry criteria, predict if the PFs molecule is chiral or non-chiral.arrow_forwardIdentify the atom that has each ground-state electron configuration. Q.) 1s 2 2s 2 2p4arrow_forward
- Akiloenreachor is what 3. मेगा ाarrow_forwardWhat is the correct electron configuration for the molecularorbital of B2+? (5B) diboronearrow_forwardEnergy 4. Draw the MO diagram for the following compound where each wave function is represented as the sum of p-orbitals. Include all nodes. Fill in the orbitals on the right of the diagram with the appropriate number of electrons.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning