
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 3, Problem 12CTQ
Interpretation Introduction
Interpretation: Lewis structure that corresponds to orbital description shown below should be drawn.
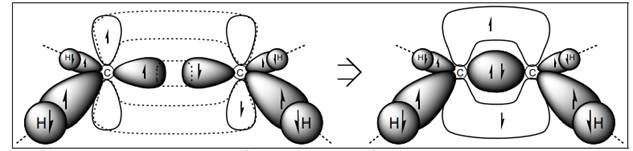
Concept introduction: Lewis structures depict covalent bonds and describe valence electrons are present in the molecule.
The sequence that leads to Lewis structure of the molecule is as follows:
- Identify central atom and arrange various other atoms around it. This atom so chosen is the least electronegative one.
- Estimate the total valence electrons.
- Firstly place single bond between each pair.
- The remaining electrons can be allocated lone pairs to satisfy the octet rule for each atom.
Bonds formed due to head-to-head overlap are termed sigma bond while ones formed by sideways or lateral overlap are named pi-bonds.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Click on the atom you would expect to have the greatest valence electron density (i.e. partial negative charge).
need to choose one
Consider the Lewis structure shown for thionitromethane.
Draw the major resonance structure for the compound shown; include lone pairs of electrons, formal charges, and condensed hydrogen atoms (located in the More menu). Then draw curved arrows to show how this can be converted to the Lewis structure given.
a) Draw the formal charges to the molecule below. Label atleast two functional groups found in this molecule. Identify and circle the π bonds.
b) Draw a curly arrow notation to show how the electrons are redistributed to show a new resonance structure in which the formal charge has moved to a different heteroatom.
Chapter 3 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 3 - Prob. 1CTQCh. 3 - What neutral atom is represented by the electron...Ch. 3 - Prob. 3CTQCh. 3 - Consider any one of the four identical hybrid...Ch. 3 - Prob. 5CTQCh. 3 - Prob. 6CTQCh. 3 - Prob. 7CTQCh. 3 - Prob. 8CTQCh. 3 - Prob. 9CTQCh. 3 - Prob. 10CTQ
Ch. 3 - On the left side of Figure 3.6, label the areas...Ch. 3 - Prob. 12CTQCh. 3 - Prob. 13CTQCh. 3 - Prob. 14CTQCh. 3 - Prob. 15CTQCh. 3 - Now consider the fully formed molecule on the...Ch. 3 - Prob. 1ECh. 3 - Explain why the two molecules below cannot...Ch. 3 - Prob. 3ECh. 3 - Consider the incomplete orbital representation of...Ch. 3 - Consider the following orbital representation of...Ch. 3 - Summarize how one determines the hybridization...Ch. 3 - Explain what is wrong with each of the following...Ch. 3 - Prob. 8ECh. 3 - Prob. 9ECh. 3 - Complete the following tables, and memorize their...Ch. 3 - Draw orbital representations of bonding in water...Ch. 3 - Draw electron configuration diagrams for carbon in...Ch. 3 - Prob. 13E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give typed full explanation Look at figure 3-22 that shows the electron density that occurs abound the Si-O bond. This electron density map gives the "shape" of the O and Si atoms when they are bonded together. Think about the answer in Q9 and choose the best response below: (Select answer choice) a. This figure shows that the Si and O atoms, when they bond together, do not form spheres, which is due to the fact that the Si-O bond is strongly covalent and these shared electrons affect atomic shape. This change in shape limits the applicability of Pauling's Coordination principle since that principle is based on the geometry of perfect spheres. b. This figure shows that the Si and O atoms, when they bond together are close to perfect spheres, which is due to the fact that the Si-O bond is strongly covalent. This figure shows that Pauling's Coordination principle should apply very precisely to any substance that contains Si-O bonds c. This figure shows that the Si and O atoms, form in a…arrow_forwardWhat is the name of this type of molecular structure?? I need help answering this question because I don’t know what format it’s calledarrow_forwardquestion. Draw a Lewis structure for the C,H, molecule using the connectivity shown in the spacefilling model in the window.arrow_forward
- It is impossible to draw a legitimate Lewis structure of a neutral NH4 molecule. Hypothetically,how many valence electrons would such a neutral NH4 molecule have ifit could exist? a. The +1 cation, NH4+ , does exist. How many valence electrons does one NH4+ ion have? b. Draw the Lewis structure for NH4+arrow_forwardWhich elements on the periodic table (other than H) are likely to form a+1 cation?arrow_forwardConsider the polarization of the C=O bond in the molecule acetone a. (E) Which atom (C or O) is expected to have a greater electron density? b. (E) Add a + and to this bond to emphasize its polarization.arrow_forward
- On the left side of Figure 3.6, label the areas shown with a dotted line where... one bond can form. one bond can form.arrow_forwardanswer 3 and 5. PLS MAKE IT LIKE THIS WAY SO THAT I UNDERSTAND IT IONIC YES OR NO POLAR YES OR NO NON POLAR YES OR NO IMF EXIST IS..... NO EXPLANATION NEEDED.arrow_forwardI got wrong for draw the Lewis and how ?arrow_forward
- Can I have help drawing the Lewis structure with a minimilized formal charge in the the attached image.arrow_forwarda) Draw the Lewis structure for the molecule on the left (labeled as Molecule A above). Draw the Lewis structure which has minimum formal charges. b) Draw the correct Lewis structure for the molecule on the right (labeled as Molecule B above). Draw the Lewis structure which has minimum formal charges. c) Select the three TRUE statements from those provided below. The molecule on the right (Molecule B) is planar (all atoms lie within the same plane). The molecule on the left (Molecule A) is planar (all atoms lie within the same plane). The molecule on the right (Molecule B) has polar bonds. The molecule on the left (Molecule A) has polar bonds. We can distinguish between the two molecules (Molecule A and Molecule B) based upon their dipole moment.arrow_forwardQ/ For the Lewis structure below…• Draw all of its important resonance structures. Show all charges and lone pair electrons. Use curved arrows to show the flow of electrons leading to each successive structure.• Draw the resonance hybrid. Label the average bond order and average chargesarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning