
Concept explainers
(a)
Interpretation: Dotted lines to show
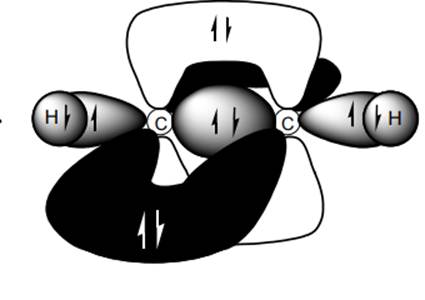
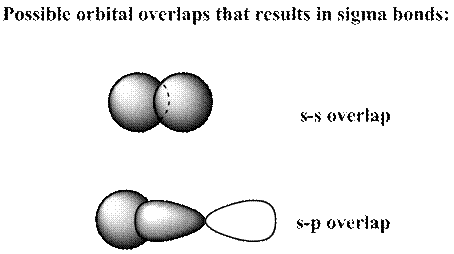
Concept introduction: Bonds formed due to head-to-head overlap are termed sigma bond while ones formed by sideways or lateral overlap are named pi-bonds.
A single bond has one
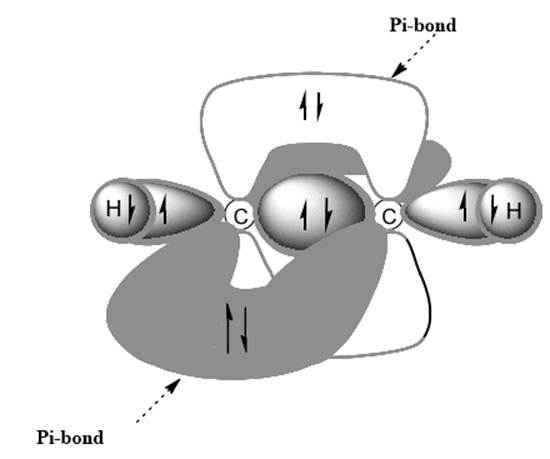
(b)
Interpretation: Dotted lines to show
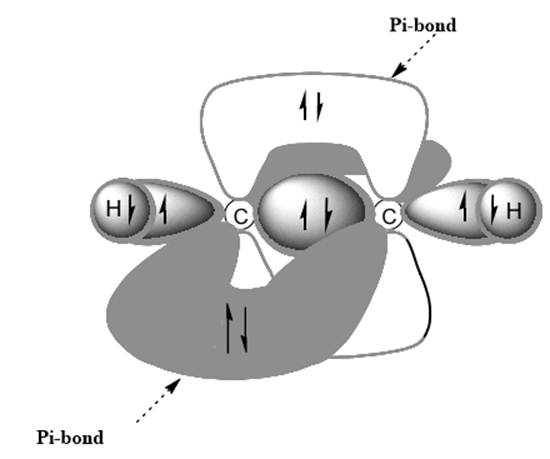
Concept introduction: Bonds formed due to head-to-head overlap are termed sigma bond while ones formed by sideways or lateral overlap are named pi-bonds.
A single bond has one
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Organic Chemistry: A Guided Inquiry
- A student draws the picture of ammonia (NH3) in the box below, left, and predicts it will be a flatmolecule with HNH bond angles of exactly 120°. Unfortunately, the student left something out. a. What did the student omit from his drawing? b. What is the actual HNH bond angle of ammonia (based on the draw g above, right)? c. Explain why water, ammonia, and methane (shown below) all have about the same bondangles (close to 109.5°) even though they have different numbers of bonds.arrow_forwardNow consider the fully formed molecule on the right side of Figure 3.7. a. Draw a Lewis structure of this molecule. b. Identify orbital representations of the two bonds and three bonds in Figure 3.7, andmatch these with representations of bonds on the Lewis structure you just drew.arrow_forwarda) Draw the Lewis structure for CF3. Show credit. b) What is the shape or geometry at the center atom? c) What is the bond angle at the center atom? Enter a plain number, but include a "<" sign in front if needed. d) What is the hybridization at the center atom? Enter without superscripts. For instance, if it were sp, you would type "sp3". e) Is the molecule polar or nonpolar overall? Type "polar" or "nonpolar".arrow_forward
- 1. Do all of the following for each molecule or ion. a. Draw the Lewis Dot structure that minimizes formal charges. b. Draw the 3-D structure. c. Determine the Hybridization designation of the central atom. d. Determine the VSEPR designation. e. State what is its Molecular shape. f. Determine whether it is Polar (P) or Nonpolar (N). g. Determine the number of sigma (0) and pi (0) bonds in each structure А. РОд 3- B. SeF4 C. CH,O D. BrF5 Е. СН2arrow_forwardw and name the correct orbitals involved when a Si atom and four F atoms come ether to form SİF4. Your drawing should clearly show "before" and "after" forming the ds. Draw the orbitals as clearly as you can, but you only have to show the orbitals for E of the Si-F bonds being formed -- don't draw all four.arrow_forward1. a) Sketch the Lewis structure and name the molecular VSEPR shape for CCl4 b) What is the hybridization for the C atoms in C2H2? Show your work. (Start with a Lewis structure. This molecule has a skeleton of HCCH.) c) Sketch an s orbital. (This is meant to be a simple question. Don't sweat it!) d) Sketch a p orbital of your choice. Indicate the phase and the cartesian directions (x, y, z axes).arrow_forward
- The next part of the problem is to draw the resonance hybrid. (Exclude formal charges) I’m not sure where to put the dotted resonance lines of the hybrid structure.arrow_forwardQ/ For the Lewis structure below…• Draw all of its important resonance structures. Show all charges and lone pair electrons. Use curved arrows to show the flow of electrons leading to each successive structure.• Draw the resonance hybrid. Label the average bond order and average chargesarrow_forward5. Follow the curve arrows in the image below and draw the correct second resonan structure.arrow_forward
- a) Draw the Lewis structure for CH,F2. lit. b) What is the shape or geometry at the center atom? c) What is the bond angle at the center atom? Enter a plain number, but include the "<" sign in front if needed. d) What is the hybridization at the center atom? Enter without superscripts. For instance, if it were sp', you would type "sp3". e) Is the molecule polar or nonpolar overallI? Type "polar" or "nonpolar".arrow_forwardDescribe the orbitals that are mixing to make the bond that the thicker arrow is pointing to. (formatting is shown for the C:O double bond pointed to by the thinner arrow)arrow_forwardHelp me pleasearrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
