
Organic Chemistry (8th Edition)
8th Edition
ISBN: 9780134042282
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 28, Problem 34P
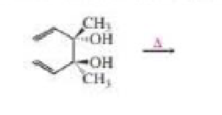
When the following compound is heated, a product is formed that shows an infrared absorption band at 1715cm-1. Draw the structure of the product.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Reaction of (CH3)3CCHO with (C6H5)3P=C(CH3)OCH3, followed by treatment with aqueous acid, affords R (C7H14O). R has a strong absorption in its IR spectrum at 1717 cm−1 and three singlets in its 1H NMR spectrum at 1.02 (9 H), 2.13 (3 H), and 2.33 (2 H) ppm. What is the structure of R? We will learn about this reaction in Chapter 18.
When the following compound is heated, a product is formed that shows an infrared absorption band at 1715 cm-1. Draw the structure of the product
As reaction of (CH3)2CO with LIC≡CH followed by H2O affords compound D, which has a molecular ion in its mass spectrum at 84 and prominent absorptions in its IR spectrum at 3600−3200, 3303, 2938, and 2120 cm−1. D shows the following 1H NMR spectral data: 1.53 (singlet, 6 H), 2.37 (singlet, 1 H), and 2.43 (singlet, 1 H) ppm. What is the structure of D?
Chapter 28 Solutions
Organic Chemistry (8th Edition)
Ch. 28.1 - Prob. 1PCh. 28.2 - Prob. 2PCh. 28.2 - Prob. 3PCh. 28.2 - Give a molecular orbital description for each of...Ch. 28.3 - Prob. 5PCh. 28.3 - Prob. 6PCh. 28.3 - Prob. 7PCh. 28.3 - Prob. 8PCh. 28.4 - Prob. 10PCh. 28.4 - Prob. 11P
Ch. 28.5 - Prob. 12PCh. 28.5 - a. Draw the product of the following reaction: b....Ch. 28.5 - Prob. 14PCh. 28.5 - Prob. 15PCh. 28.5 - Prob. 17PCh. 28.5 - Prob. 18PCh. 28.6 - Prob. 19PCh. 28.6 - Explain why the hydrogen and the methyl...Ch. 28.6 - Chorismate mutase is an enzyme that promotes a...Ch. 28.7 - Convince yourself that the TE-AC method for...Ch. 28 - Draw the product of each of the following...Ch. 28 - Draw the product of each of the following...Ch. 28 - Prob. 25PCh. 28 - Show how norbornance can be prepared from...Ch. 28 - Prob. 27PCh. 28 - Prob. 28PCh. 28 - Draw the product of each of the following...Ch. 28 - Prob. 30PCh. 28 - Prob. 31PCh. 28 - Prob. 32PCh. 28 - Prob. 33PCh. 28 - When the following compound is heated, a product...Ch. 28 - Prob. 35PCh. 28 - Propose a mechanism for the following reaction:Ch. 28 - Prob. 37PCh. 28 - Prob. 38PCh. 28 - Prob. 39PCh. 28 - Prob. 40PCh. 28 - If isomer A is heated to about 100 C, a mixture of...Ch. 28 - Propose a mechanism for the following reaction:Ch. 28 - Prob. 43PCh. 28 - A student found that heating any one of the...Ch. 28 - Prob. 45PCh. 28 - Prob. 46PCh. 28 - Prob. 47P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- As we will learn in Chapter 20, reaction of (CH3)2CO with LiC ≡ CH followed by H2O affords compound D, which has a molecular ion in its mass spectrum at 84 and prominent absorptions in its IR spectrum at 3600–3200, 3303, 2938, and 2120 cm. D shows the following 1H NMR spectral data: 1.53 (singlet, 6 H), 2.37 (singlet, 1 H), and 2.43 (singlet, 1 H) ppm. What is the structure of D?arrow_forwardAs we will learn in Chapter 17, reaction of (CH3)2CO with LIC≡CH followed by H2O affords compound D, which has a molecular ion in its mass spectrum at 84 and prominent absorptions in its IR spectrum at 3600−3200, 3303, 2938, and 2120 cm−1. D shows the following 1H NMR spectral data: 1.53 (singlet, 6 H), 2.37 (singlet, 1 H), and 2.43 (singlet, 1 H) ppm. What is the structure of D?arrow_forwardTreatment of isobutene [(CH3)2C = CH2] with (CH3)3CLi forms a carbanion that reacts with CH2=O to form H after water is added to the reaction mixture. H has a molecular ion in its mass spectrum at m/z = 86, and shows fragments at 71 and 68. H exhibits absorptions in its IR spectrum at 3600–3200 and 1651 cm−1, and has the 1H NMR spectrum given below. Whatis the structure of H?arrow_forward
- The treatment of (CH3)2C = CHCH2Br with H2O forms B (molecular formula C5H10O) as one of the products. Determine the structure of B from its H NMR and IR spectra.arrow_forwardWhen 2-bromo-3,3-dimethylbutane is treated with K+ −OC(CH3)3, a single product T having molecular formula C6H12 is formed. When 3,3dimethylbutan-2-ol is treated with H2SO4, the major product U has the same molecular formula. Given the following 1H NMR data, what are the structures of T and U? Explain in detail the splitting patterns observed for the three split signals in T. 1H NMR of T: 1.01 (singlet, 9 H), 4.82 (doublet of doublets, 1 H, J = 10, 1.7 Hz), 4.93 (doublet of doublets, 1 H, J = 18, 1.7 Hz), and 5.83 (doublet of doublets, 1 H, J = 18, 10 Hz) ppm 1H NMR of U: 1.60 (singlet) ppmarrow_forwardTreatment of ketone A with ethynyllithium (HC≡CLi) followed by D3O+ afforded a compound B of molecular formula C12H13DO3, which gave an IR absorption at approximately 1715 cm−1. What is the structure of B and how is it formed?arrow_forward
- : Treatment of (CHa)CHCH(OH)CH,CH3 with TSOH affords two products (M and N) with molecular formula CgH12. The 'H NMR spectra of M and N are given below. Propose structures for M and N and draw a mechanism to explain their formation. 1H NMR of M 3H 1H NMR of N 3H 3H 3 H 1H 3 H 2 H 2H 2H 8 7 6 4 1 0 9 8. 2 1 ppm ppm 4.arrow_forwardA compound of formula C6H10O2 shows only two absorptions in the proton NMR: a singlet at 2.67 ppm and a singlet at2.15 ppm. These absorptions have areas in the ratio 2:3. The IR spectrum shows a strong absorption at 1708 cm-1. Proposea structure for this compound.arrow_forwardTreatment of 2-methylpropanenitrile [(CH3)2CHCN] with CH3CH2CH2MgBr, followed by aqueous acid, affords compound V, which has molecular formula C7H14O. V has a strong absorption in its IR spectrum at 1713 cm−1, and gives the following 1H NMR data: 0.91 (triplet, 3 H), 1.09 (doublet, 6 H), 1.6 (multiplet, 2 H), 2.43 (triplet, 2 H), and 2.60 (septet, 1 H) ppm. What is the structure of V? We will learn about this reaction in Chapter 20.arrow_forward
- Benzonitrile (C6H5CN) is reduced to two different products depending on the reducing agent used. Treatment with lithium aluminum hydride followed by water forms K, which has a molecular ion in its mass spectrum at 107 and the following IR absorptions: 3373, 3290, 3062, 2920, and 1600 cm-1. Treatment with a milder reducing agent forms L, which has amolecular ion in its mass spectrum at 106 and the following IR absorptions: 3086, 2820, 2736, 1703, and 1600 cm-1. L shows fragments in its mass spectrum at m/z = 105 and 77. Propose structures for K and L and explain how you arrived at your conclusions.arrow_forwardHeating the compound shown here at 225–235 °C for 8 h produced a new compound with the formula C23H16N,0. The mechanism for this reaction is believed to consist of a [4+2] cycloaddition followed by a [4+2] cycloelimination. The 'H NMR spectrum of the product contained the following signals: 8 8.33–8.25 and 7.58–6.97 (m, 14 H), 5.46 (s, 2 H). (a) Draw the mechanism for this reaction. (b) Draw the product. C6H5 C6H5 N. ? C23H16N20arrow_forwardTreatment of compound E (molecular formula C4H8O2) with excess CH3CH2MgBr yields compound F (molecular formula C6H14O) after protonation with H2O. E shows a strong absorption in its IR spectrum at 1743 cm-1. F shows a strong IR absorption at 3600–3200 cm-1. The 1H NMR spectral data of E and F are given. What are the structures of E and F?Compound E signals at 1.2 (triplet, 3 H), 2.0 (singlet, 3 H), and 4.1 (quartet, 2 H) ppmCompound F signals at 0.9 (triplet, 6 H), 1.1 (singlet, 3 H), 1.5 (quartet, 4 H), and 1.55 (singlet, 1 H) ppmarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY