
Interpretation:
The correct product should be identified for the given [1, 3] sigmatropic rearrangement reaction.
Concept introduction:
In a sigmatropic reaction “ one new sigma-bond is formed as another breaks.”
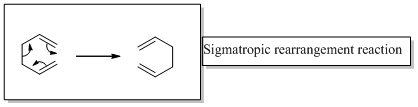
Sigmatropic rearrangement reactions are designated with digits. For example a [1, 3] sigmatropic rearrangement describe a reaction in which the residue migrates from position 1 to position 3.
[3,3] sigmatropic rearrangement of a 1,3-diene known as Cope rearrangement reaction.
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for sigmatropic rearrangement reactions are listed below


Carbon migrating with one lobe of its p orbital interacting
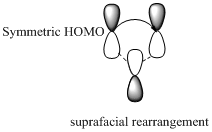

Carbon migrating with both lobe of its p orbital interacting

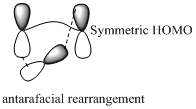
of configuration is the inversion of a chiral center in a molecule in a
Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
Organic Chemistry (8th Edition)
- Examine the following pericyclic reactions.Tell whether it is an electrocyclic reaction, a cycloaddition reaction, or a sigmatropic rearrangement.arrow_forwardDraw the product (s) of the following reaction. If the formation of one product is preferred over the other stereo specifically or stereoselectively, then draw Newman projections to show why. Determine the stereochemistry of the product (s).arrow_forwardThe molecule in the box is the product of a diels alder reaction. Which of these is the dienophile in that reaction ? A B C D or E?arrow_forward
- Given that an E2 reaction proceeds with anti periplanar stereochemistry, draw the products of each elimination. The alkyl halides in (a) and (b) are diastereomers of each other. How are the products of these two reactions related? Recall from Section 3.2A that C6H5– is a phenyl group, a benzene ring bonded to another group.arrow_forward1. Draw the product of each of the following [3,3] sigmatropic rearrangements, including its stereochemistry. Ph CH3 (a) H3C. (b) Ph CH3 (c) H3C CO₂CH3 CH3 H (d)arrow_forwardWhat will be the correct stereochemistry of the product?arrow_forward
- Given that an E2 reaction proceeds with anti periplanar stereochemistry, draw the products of each elimination. The alkyl halides in (a) and (b) are diastereomers of each other. How are the products of these two reactions related? Recall from Section 3.2A that C6H5 −is a phenyl group, a benzene ring bonded to another group.arrow_forwardDraw the product(s) that you expect for the following reaction. Show stereochemistry, if applicable, by clearly drawing one wedged, one hashed, and two plane bonds per stereocenter.arrow_forwardDraw a reaction mechanism illustrating the transition state structure and stereochemistry of the resulting product (if any).a. (R)-2-bromobutane + -OCH3 → (SN2)b. (S)-3-bromo-3-methylhexane + methanol → (SN1)c. 2-Chloro-2-methylhekxane + -OH → (E1)arrow_forward
- addition of hbr to a double bond with an ether (-or) substituent occurs regiospecifically to give a product in which the Br OR are bonded to the same carbon. Draw the two possible carbocation intermediates in this electrophilic addition reaction,and explain using resonance why the observed product is formed.arrow_forwardGiven that an E2 reaction proceeds with anti periplanar stereochemistry, draw the products of each elimination. The alkyl halides in (a) and (b) are diastereomers of each other. How are the products of these two reactions related? Recall from Section 3.2A that CgHs- is a phenyl group, a benzene ring bonded to another group. "OCH,CH, b. CgHgC CH Br OCH,CH3 a. Brarrow_forward3) Explain how and why the reaction below results in the observed regiochemistryand/or stereochemistry.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





